Immunogenic Cell Death: Bench Comes to Bedside…With Caveats
Washington DC VA Medical Center
mmunologic therapies for cancer wax and wane in popularity. Interest is now waxing for monoclonal antibody-based checkpoint inhibitor therapy because of incredible responses in patients with advanced lung, kidney, and melanoma cancers. In addition, the use of oncologic viruses such as IMLYGIC (talimogene laherparepvec or T-VEC) has spurred a renewed enthusiasm for such viruses as immunologic agents. Radiation and phototherapy also seem to drive immune responses that cause untreated lesions to regress (the abscopal effect). Checkpoint inhibitors are now standard of care in first line treatment of Non-Small Cell Lung Cancer. However, recent data indicate that checkpoint inhibitor therapy appears to be most successful in patients whose malignancies have a high Tumor Mutational Burden to create neoantigens, numerous inflammatory and immune cells that infiltrate tumors to make them “Hot,” and evidence of an immune response by the expression of PD-L1, a checkpoint inhibitor ligand.
The enhanced response rate to T-VEC when given intralesionally along with persuasive preclinical data suggest that certain cancers, like colon cancer, may show a much higher response rate if the therapy is administered intralesionally as compared to the usual parenteral administration. A resulting higher Immunogenic Cell Death (IHC) could be a great benefit to the cancer patient.
T-VEC for intralesional treatment of metastatic cutaneous melanoma is an important example of why immunology may be important. About a quarter of patients have regression of uninjected lesions as well as those who have been injected when T-VEC is injected intratumorally into cutaneous metastases of melanoma. FDA has considered this as evidence of possible immune-mediated regression. It is difficult to demonstrate that these unexpected regressions result from an immune response, and, in some cases, may be delayed responses to prior therapies. Is there a possible explanation for these phenomena where a treatment may cross-prime adaptive immune responses to tumor antigens?
CMAX
Maximum concentration of an agent in vivo, usually in the central compartment
DACH
1,2-diaminocyclohexane, the carrier ligand for Oxaliplatin
ER
Endoplasmic Reticulum
IC50
50 percent Inhibitory Concentration of a drug measured in vitro in a cytotoxicity assay
ICD
Immunogenic Cell Death
T-VEC
Oncolytic virus talimogene laherparepvec
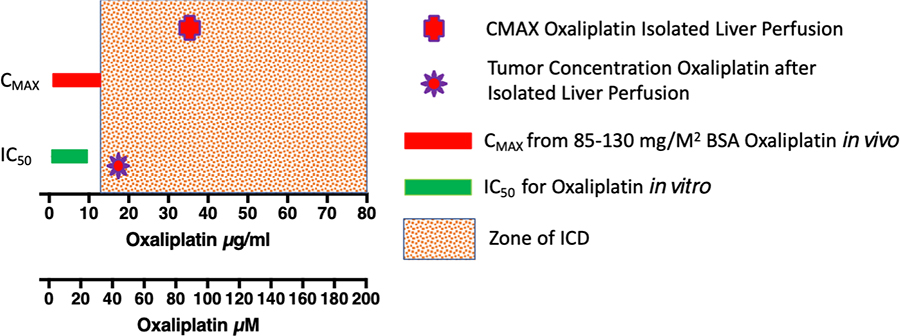
The externalization of Calreticulin with the ER luminal contents occurs before loss of membrane permeability and the externalization of Annexin V. Also, this exocytosis is associated with the release of ATP along with Danger Associated Molecular Pattern (DAMP) molecules, such as HMBG1 and HSP70. Once outside the cell, Calreticulin serves as an “eat me” signal with ATP that attracts dendritic and other antigen-presenting cells to the dying cancer cell. The DAMPs then provide “take me” signals that load dendritic cells and activate their ability to activate naive T- and B-cells. As shown in Figure 1, the concentration to induce ICD activity is generally greater than the CMAX for such chemotherapeutic agents as Oxaliplatin.
This process of ICD makes “Cold” tumors “Hot.” In our pre-clinical experience, ICD caused a four-fold increase in infiltrating CD3+ T-cells that were naive with decreased expression of PD-1 on CD8+ T-cells compared to infiltrating CD8+ T-cells in untreated tumors that were PD-1+ and exhausted. ICD in the established CT26 tumors after a single intratumoral injection of Oxaliplatin and oncolytic virus inhibited tumor growth in all mice with complete regression in 40 percent of mice, cytotoxic lymphocytes in draining nodes and transplantation resistance that rejected a viable tumor cell challenge. All with just a single intratumoral injection and without the addition of checkpoint inhibitors.
However, there is a major caveat to the induction of ICD. Since ICD requires multiples of the IC50 for the inducing drug, it seems unlikely that standard systemic chemotherapy will actually induce ICD in the majority of patients. As shown in Figure 1 for Oxaliplatin, the CMAX after the systemic administration of either 85 or 130 mg/M2 is barely into the region that causes ICD in tumor tissue. The CMAX is measured in the central compartment of blood where the half-life is 15-25 minutes. The hepatic clearance from the central compartment is not described in the literature for systemic administration, so it is difficult to predict what the intratumoral concentration of Oxaliplatin will be.
A small Phase 1 trial of isolated hepatic perfusion in Pittsburgh achieved a CMAX three times that of systemically administered Oxaliplatin, with a concentration of Oxaliplatin in metastases that was just inside the range of Oxaliplatin concentration that causes ICD (Figure 1). Oxaliplatin, like other agents, has a limited time of activity before its effectiveness is lost. It and its metabolites of mono and dichloro DACH platin and diaquo DACH platin are active for only four hours before being inactivated by conjugation and protein binding. Thus, systemic administration of Oxaliplatin would need to be increased by more than three-fold to induce ICD within liver metastases. Since other cytotoxic ICD inducers like Oxaliplatin are near their maximum tolerated dose, it is not likely that systemic administration will stimulate an immune response to the tumor by itself that causes regression of an established disease.
Regional delivery may be the answer. In seven patients in the Pittsburgh Phase I trial with unresectable colorectal carcinoma liver metastases there was one complete response, one progressive disease, one stable disease, and four partial responses. With techniques like transarterial chemoembolization or image-guided intratumoral injection, it is likely that the high intratumoral concentration of Oxaliplatin and similar drugs may be obtained without causing systemic toxicity. Work with nanoparticles that target tumors with a high enough concentration to activate ICD without causing systemic toxicity are also likely to cross-prime patients’ immune responses to their tumors.
In summary, ICD is a product of the bench, but its usefulness needs to be put into the context of clinical care. ICD requires intratumoral concentrations of inducers that may seem too high for systemic administration. However, the potential benefits of ICD may be reaped with advances in targeted delivery of ICD inducers to malignant lesions. Finally, if improvements in regional therapy with ICD inducers makes “Cold” tumors “Hot,” then nearly 60 percent of all cancer patients will become candidates for checkpoint inhibitor therapy.

