Developing a Sponsor-Specific Clinical Trial Diversity Playbook
Acclinate, Inc.
here is increasing agreement that aiming for health equity through inclusive clinical research is in the best interest of patients. This renewed focus from research sponsors on diversifying their clinical trials has also been driven by new laws (i.e., the December 2022 Food and Drug Omnibus Reform Act – FDORA, sections 3601-3604) and regulatory guidance (i.e., the April 2022 FDA Draft Guidance to Industry on Diversity Plans to Improve Enrollment of Participants from Underrepresented Racial and Ethnic Populations in Clinical Trials) that require submission of diversity plans to FDA prior to the start of pivotal studies.
This article provides novel guidance to research sponsors on how to use “affective” (i.e., emotion-based) trust, regulatory guidance, organizational assessment, and therapeutic area (TA) considerations to develop a sponsor- and TA-specific playbook for addressing clinical trial diversity.
The Foundation
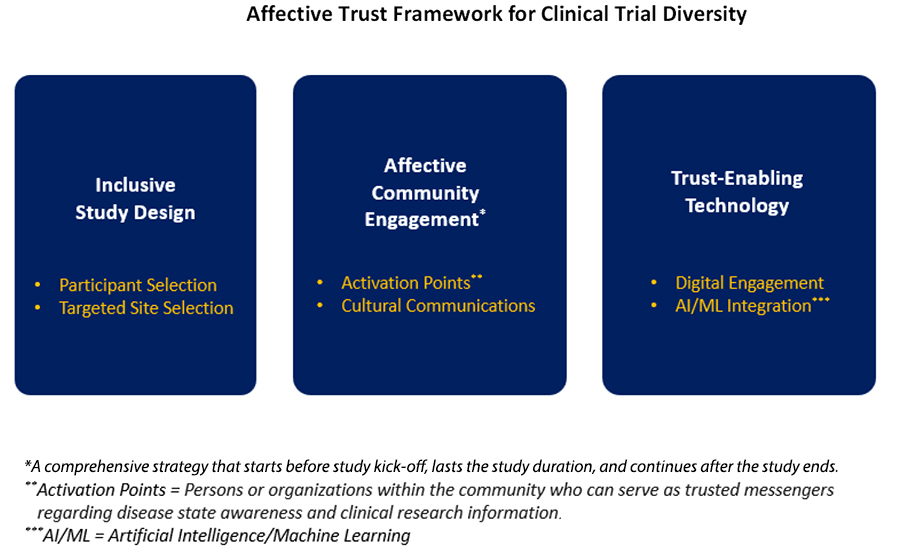
We have designed the Affective Trust Framework for Clinical Trial Diversity, which serves as the foundation for the clinical trial diversity playbook. The framework consists of three key pillars (see Figure 1) focused on elements of inclusive study design, sustained (“affective”) community engagement with historically excluded populations, and trust-enabling technology. At its core, this framework recognizes that for research sponsors to impactfully engage communities of color, they must first establish emotion-based affective trust.
Affective trust is steeped in genuine concern and personal goodwill toward underrepresented populations, whereas cognitive trust is based solely on rationality, data, and evidence. Cultivating affective trust requires sponsors to build relationships with historically excluded populations by seeking to understand that community’s lived experiences, by engaging with culturally competent community influencers who can share information about various disease states and research opportunities, and by investing in the community’s general health and wellness. A mix of in-person engagement plus trust- enabling technology (e.g., social media, email/text campaigns, predictive analytics) serves to sustain these relationships by facilitating broader outreach and identifying which community members may actually be willing to participate in clinical trials. Without affective trust, communities of color are less likely to participate in or maintain involvement in clinical research.
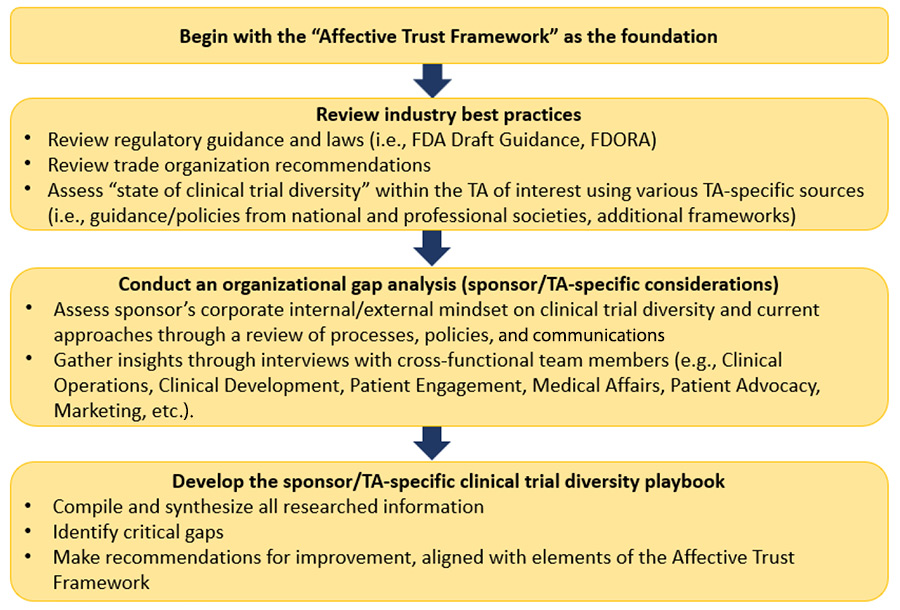
The Process
An Oncology Use Case
This use case describes the development of a playbook for a research sponsor with oncology as the TA of interest. Assessing these FDA guidance documents, FDORA, and “industry best practices” from such trade organizations as the Pharmaceutical Research and Manufacturers of America and the Biotechnology Innovation Organization, and combining them with clinical trial diversity recommendations from the American Association for Cancer Research, American Society of Clinical Oncology, Association of Community Cancer Centers, and the American Society of Hematology, yields recommendations specific to improving diversity in oncology clinical trials that can be layered on top of the initial Affective Trust Framework.
Recommendations may include suggestions on which key components to consider for diversity plan submission to the FDA, as well as advice for improving culturally tailored health literacy, for leveraging digital means and community partnerships (i.e., activation points) to extend reach, and for upskilling community oncology practices to become research sites.
In addition, an organizational gap analysis of the sponsor’s current approach to trial diversity, including a review of the sponsor’s internal/external prioritization of it and feedback from employee interviews, results in sponsor-specific recommendations for achieving diversity in the sponsor’s oncology clinical trials. Outputs from this organizational gap analysis may include revisions to the sponsor’s clinical development planning templates, addition of race/ethnicity demographic questions to study-specific site feasibility questionnaires, consideration of “up-and-coming” oncology researchers with diverse backgrounds for protocol steering committees/investigator opportunities, and new cross-functional team collaborations to facilitate broader understanding of the sponsor’s clinical trial diversity goals within the organization.

