Around the Globe
Takeda Pharmaceutical Company Ltd.
Pfizer (China) Research & Development Co., Ltd.
he concept of DCT is attracting more and more attention in China. The paper Expert Consensus on Decentralized and Digitalized Clinical Trials, jointly written by the DIA China Digital Health Community (DHC) and Shanghai Pharmaceutical Association, was recently published (in Chinese) in the Chinese Journal of New Drugs and Clinical Remedies. DCT stands for not only decentralized clinical trial but also digitalized clinical trial in China.
Regulatory Framework
- Technical Guidance for the Design of Patient-Focused Clinical Trials (Draft) (in Chinese)
- Technical Guidance for the Implementation of Patient-Focused Clinical Trials (Draft) (in Chinese)
- Technical Guidance for Benefit-Risk Assessment of Patient-Focused Clinical Trials (Draft) (in Chinese)
All experts agreed in the consensus paper that DCT implementation requires technical/digital solutions as well as a clinical trial team to best serve the patients, and that either fully remote or hybrid remote (remote and on-site combined) can achieve the goal of patient-centricity. The paper provided a comprehensive model of the DCT digital platform.
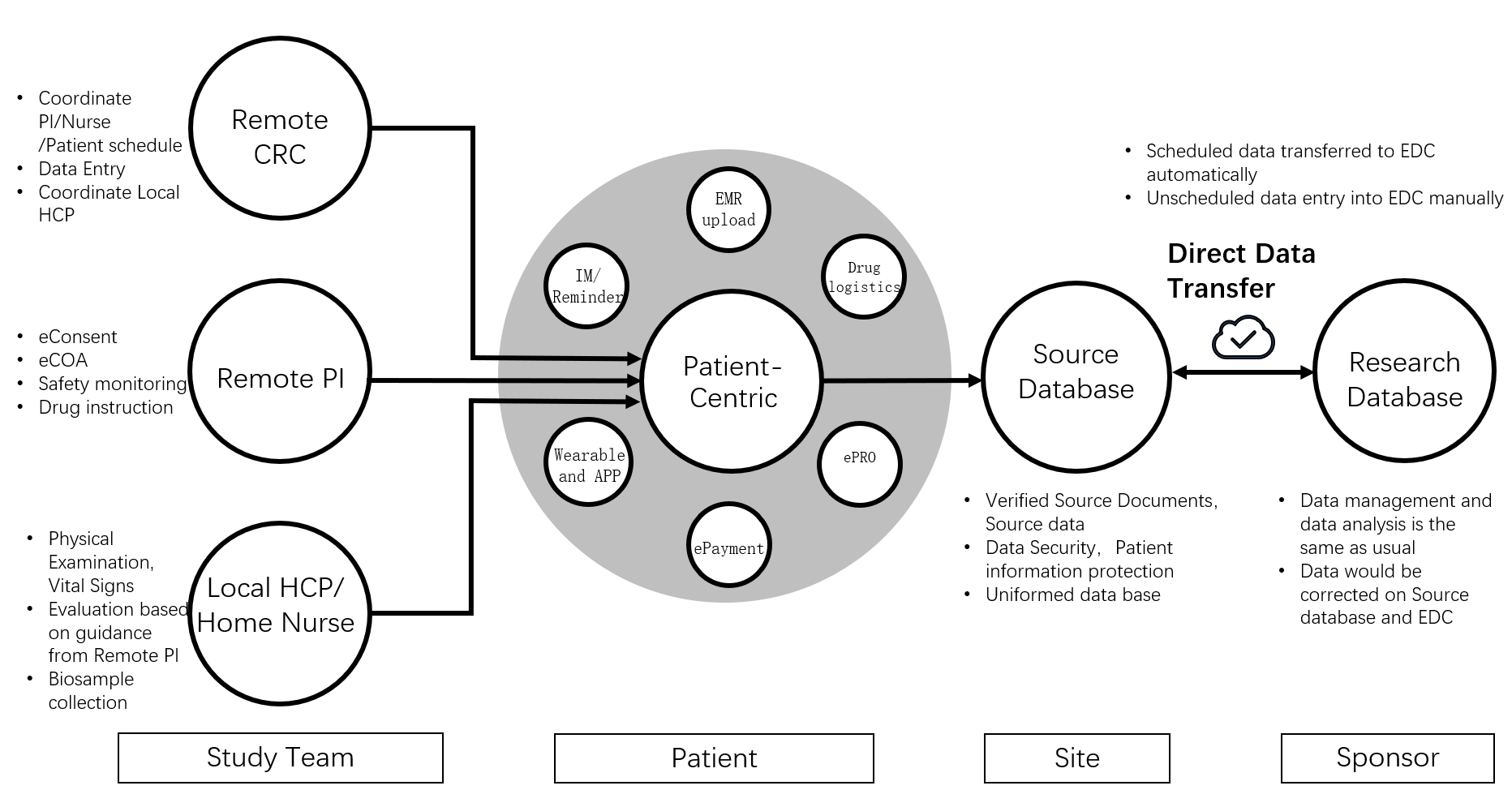
Unique advantages and different approaches in China
What are the differences between DCT implementation approaches in China and abroad?
Differences are also reflected in other aspects:
Regulatory requirements: Clinical trials in China must follow guidance from the National Medical Products Administration (NMPA) and the Human Genetic Resources Administration of China (HGRAC).
Patient preferences: Patients may prefer to go to big hospitals in big cities for medical treatments, particularly for difficult and/or complicated medical conditions.
Personal information processing: China has recently published laws and regulations to govern personal information handling, such as:
- China Cybersecurity Law (in Chinese)
- China Data Security Law (in Chinese)
- China Personal Information Protection Law (PIPL) (in Chinese)
These differences will require careful consideration particularly when regulatory submissions outside China using data generated from such trials are planned.
What are the advantages of, and obstacles to, DCTs in China?
According to the 50th Statistical Report on Internet Development in China released by the Internet Network Information Center, the number of internet users in China reached 1.051 billion, and the internet penetration rate reached 74.4%, as of June 2022. One billion users form the world’s largest and most vibrant digital society. China’s digital healthcare industry has embraced new opportunities as the nation sees broad application of digital healthcare methods and technologies. Chinese consumers have quickly embraced the rapid transition of business models from B2B (business to business) to B2C (business to consumer), which could help DCTs transition from B2B (traditional on-site trial) to B2C (conducted remotely at patient home).
Second, the regulatory environment is supportive. The newly issued Personal Information Protection Law has laid the foundation for the protection of patients’ privacy in DCTs. The NMPA has been quite open and positive toward adoption of DCT technologies.
Third, the current distribution of medical resources may indeed facilitate the growth of DCTs. In China, medical resources are relatively concentrated in major hospitals and medical centers in large and midsized cities, which often requires patients to undertake long travel to clinical trial sites. Remote approaches in the DCT model, such as telemedicine, direct-to-patients (DTP), home nursing, etc., can improve access and convenience for patients to participate in research.
Among the obstacles and challenges, the experts believe that current risk management frameworks may not be suitable for DCTs, which may raise compliance concerns for the sponsor, DCT supplier, and investigator. Therefore, it is necessary to refine the risk management framework to accurately reflect DCT-specific risks.
Additional challenges include: (1) No specific technical standards for the eConsent, eSource (electronic medical record [EMR] to electronic data capture [EDC]), and DTP aspects of DCT implementation; (2) no efficient eSource solutions for interactions between hospital information system and research database (EDC); and (3) training for DCTs is largely insufficient. Validating innovative DCT technologies, protecting patient privacy, and providing adequate support and training for both DCT patients and sites must also be further studied.
DIA China DHC thanks these five interviewees who shared their views on DCTs in China.
- Jing Zhang: Director of Phase I Clinical Research Center, Huashan Hospital
- Chen Yao: Deputy Director of Peking University Clinical Research Institute
- Yifeng Shen: Institutional Office Director, Shanghai Mental Health Center
- Zhaohua Chen: Vice President and General Manager, Global Product Development, Pfizer China
- Tong Guo: Vice President, Business Development, IQVIA Greater China.
The authors also thank, for their contributions to this work: Dong Guo (Eli Lilly & Company), Gaoyang Li (Tianjing Happy Life Tech), Kaiyan Tang (Labcorp Inc.), Juan Du (Phastar), Peili Wang (Happy Life Tech), Xiaohui Li (AstraZeneca), and Ying Tao (AstraZeneca).

