ore than 16 million adverse events have been reported to the FDA over the last 10 years, nearly four times the volume from the prior decade. This growth is driving industry-wide action to improve intake and case processing. Many companies are scaling operations and increasing efficiency with modern tools for more strategic product development. This presents both opportunities and challenges and requires fresh thinking and more nimble processes.
Simplify the Safety Systems Landscape
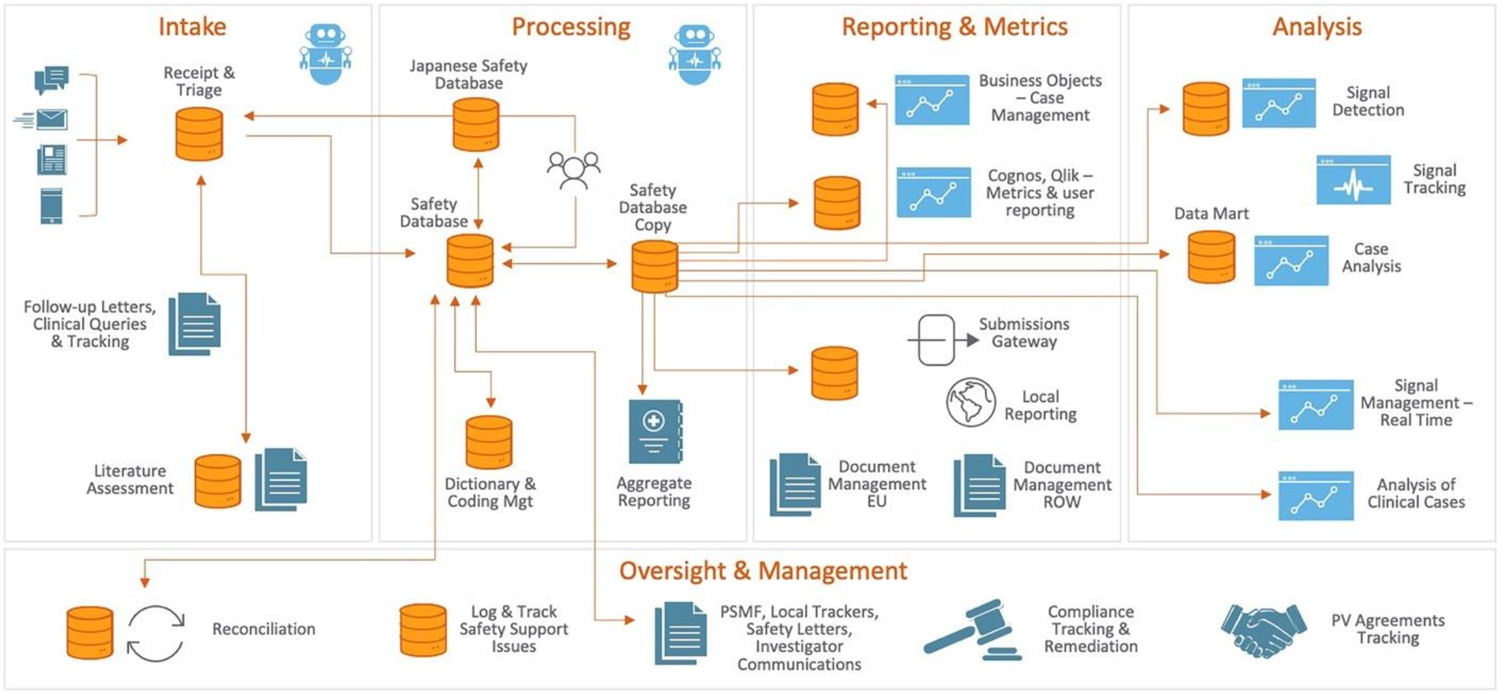
Figure 1: What does the pharmacovigilance landscape look like today? Many systems require manual intervention. (Source: Veeva Systems)
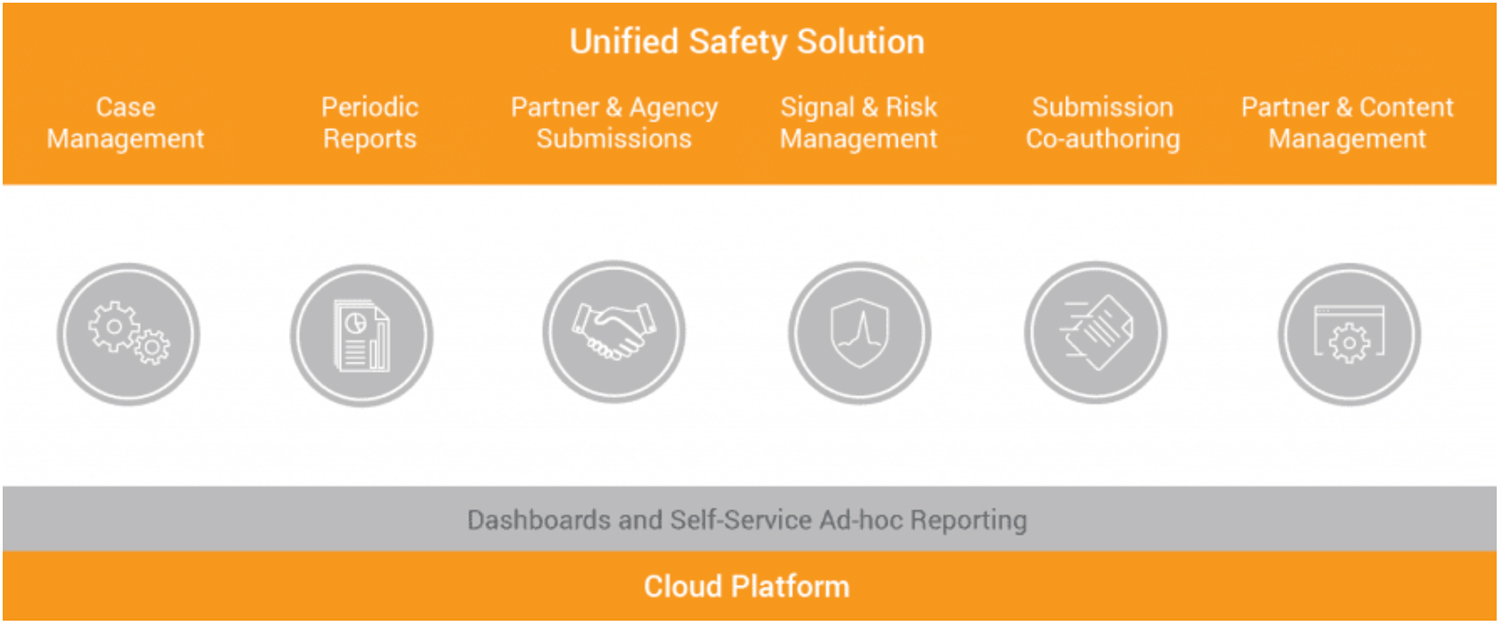
Figure 2: With key pharmacovigilance processes like case intake, case processing, and submissions in one solution, it is much easier to track and complete activities, process adverse events, and analyze data for potential signals. (Source: Veeva Systems)
Make It Easy to Keep Software Current
Cloud applications enable easy upgrades and access to new capabilities through automatic and pre-validated updates. With releases scheduled several times a year, the latest capabilities are clicks away.
To reduce the system validation burden, one should evaluate vendors that perform and document all elements of infrastructure qualification (IQ) and operational qualification (OQ) for each major version. Seamless upgrades and pre-validated systems allow pharmacovigilance teams to focus on high-value activities while maintaining compliance.
Configure, Don’t Code
Modern solutions provide the agility to create and modify business workflows with configuration, not coding. The efficiency gains reduce the administrative burden and enable greater productivity.
Think of the End User
Users should be able to effortlessly navigate through the application to find information and complete tasks. Administrators also need flexibility to support the business, with requirements like modifying security settings and changing data fields or workflows, quickly and easily.
Enable Real-time Outsourcing
“Anytime, anywhere” oversight and the ability to deliver dashboards and reports are key in an outsourced pharmacovigilance model. Sponsors should be able to access their data with real-time visibility into safety information and operational efficiency. This drives confidence that all new adverse events are captured, cases are processed on time, and challenges can be identified proactively.
Connect Data and Processes Across Functions
Solutions built on a single platform facilitate automation and access, making it easier to exchange data across teams. Seamless, cross-functional workflows minimize manual tasks such as data queries and re-entering of clinical data into safety applications. They also eliminate tedious processes like the reconciliation of serious adverse events (SAE), and quality systems capture PQCs (product quality complaints) with nonadverse events (NAEs). Companies are benefitting from this approach because it delivers a stronger data foundation across the company and increases transparency for more informed decision-making (Figure 3).
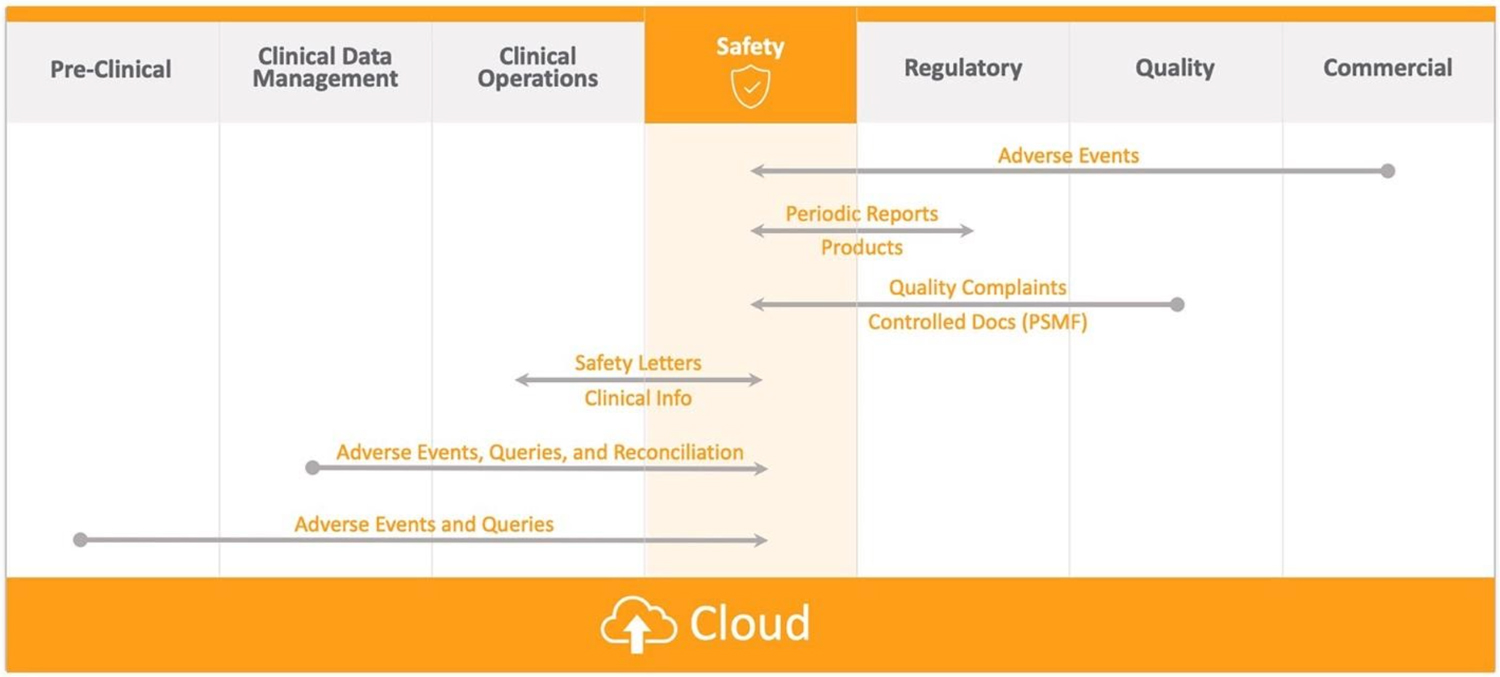
Figure 3: Connecting safety operations with functional areas across product development enables seamless information exchange and speeds execution of key pharmacovigilance processes like adverse event and queries reporting. (Source: Veeva Systems)
Plan for Growth
To simplify global submissions across different regions, look for solutions with built-in gateways, including FDA, EMA, the UK’s Medicines and Healthcare Products Regulatory Agency (MHRA), Health Canada, China National Medical Products Administration (NMPA), and Japan Pharmaceuticals and Medical Devices Agency (PMDA). This will make it easier to enter new markets through seamless reporting of adverse events to global regulators.
Drive Connected Pharmacovigilance


