Around the Globe ASEAN
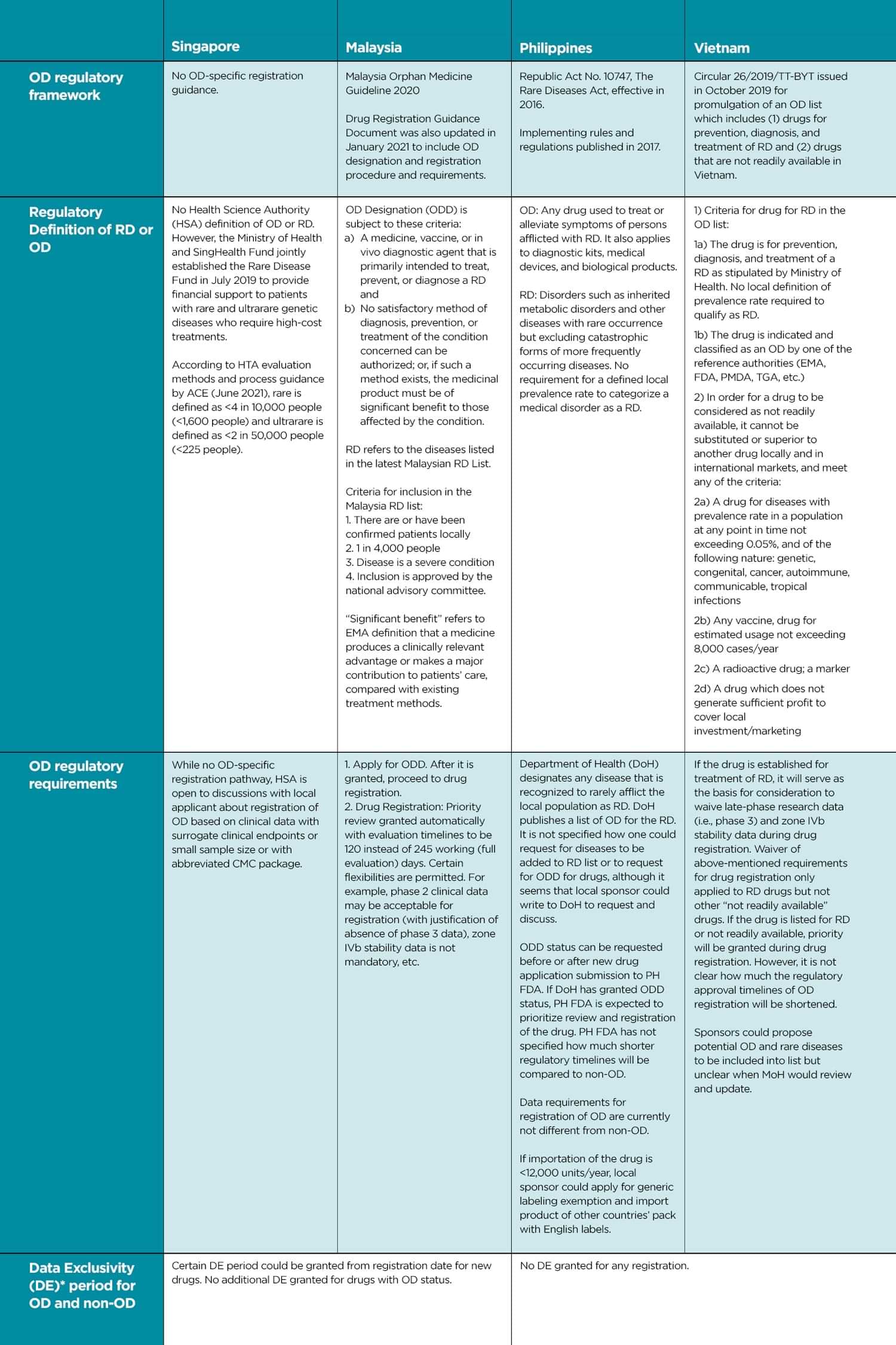
egulatory authorities such as US FDA, EMA, and PMDA have had regulatory frameworks for orphan drugs (OD) in place for decades. Upon recognizing that their existing drug development and regulatory frameworks may not be suitable for rare disease (RD) therapeutic interventions, regulators in Singapore, Malaysia, Philippines, and Vietnam have also started to initiate regulatory reforms for OD registrations. This table illustrates the current state of these reforms.
Regulators, for example, could try to better examine and understand the local prevalence and the natural history of RD to help establish a consistent definition of RD and criteria for ODD. Local registries had been established for some but not all rare diseases prevalent in these countries. It is also important for regulators to understand the treatment goal for RD patients. The growing presence of organized RD patient groups could provide meaningful input to local regulatory and healthcare policy frameworks.
It would also be critical for regulators to understand how clinical development and manufacturing size output could differ from non-OD. For example, large clinical trials with validated clinical endpoints could not be conducted, challenge of supplying country-specific packs, etc. To expedite registration, pre-submission meetings between regulators and sponsors should be encouraged to align expectations as early as possible.