Alexion Pharmaceuticals
Janssen Pharmaceuticals
Minoryx Therapeutics
Bayer
Roche
Biomarin
arlier this year, DIA held its first Regulatory Affairs Leadership Forum as part of DIA Europe 2020. The proposal was ambitious: to gather a diverse panel of people who work in different fields and discuss the value and skills that regulatory professionals bring to medicines research and development and the pharmaceutical industry of the future. This was also the first time DIA Europe was held virtually, and as such a perfect moment to reflect on the role of regulatory professionals in navigating crisis situations, such as the COVID-19 pandemic.
Key Takeaways
- Both industry regulatory teams and government regulators have been working under extreme circumstances to identify and deliver much needed solutions to this pandemic, with a collaborative spirit, constant dialogue, and “all-hands-on-deck” approaches.
- It is in times of crisis that leadership shows its mettle, and this pandemic evidenced the need for empathy, resilience, and strategic thinking to successfully address the global regulatory and public health challenges.
- Together, we must build upon the many lessons learned during this time of crisis to continuously adapt across the regulatory professions to ensure we are prepared to meet the future needs of R&D.
Regulatory Affairs Kept the Ball Rolling During the Pandemic
Current and Future Skills for Regulatory Success
- Empathy
In many development activities, regulatory professionals are the key contact with other stakeholders, particularly regarding critical marketing authorization evaluation. Regulatory professionals tell a story to others, negotiate positions, respond to public consultations, and participate in internal decision-making as to the direction of the pipeline. These interactions require a strong empathic basis to understand the views and perspectives of others and then find the middle ground in disagreements. In the digital era, these personal relationships tend to be overlooked, but they remain an important and valuable skill that regulatory professionals should nurture. - Collaboration
Once an organization sets an objective, flawless execution is a must. In this industry, nothing is flawless without a strong sense of teamwork: Regulatory professionals have always sat at interfaces, particularly between the sponsor and the regulator, and this has made them crucial catalysts for collaboration. Because they are storytellers, who can translate years of research and development into a regulatory dossier and an approved product, regulatory professionals sit at the crossroads of a high number of functions, from R&D to commercial. This collaboration remains an crucial asset of regulatory professionals as members of cross-functional teams. - Resilience and Optimism
Regulatory professionals have often been portrayed as “naysayers” due to their roles in ensuring compliance. However, they face challenges that require a resilient mindset to overcome, ranging from negative regulatory outcomes to frustrating clinical results. Programs can be shut down, and approval paths (sometimes taken for granted) can take unexpected turns. Regulatory professionals need to prepare for the worst-case scenario while hoping for the best. Some development decisions might seem unachievable, but regulatory professionals need to be the first to believe they are possible, focusing on how an outcome could be achievable rather than explaining to others why it is unlikely. - Strategic Thinking
Regulatory affairs have traditionally been a talented team for execution: We coordinate broad and diverse teams, manage large quantities of information to compile submissions, and lead complex regulatory reviews. However, the stereotype of the regulatory professional spending their days doing paperwork still thrives. Although this is a journey most regulatory professionals are on, it is necessary to reaffirm daily the strategic role we have in R&D. The more senior our R&D partners are, the clearer they view the value of the partnership with regulatory affairs. Regulatory professionals need to dedicate time and attention to strategy in their work, without being entangled in too many operational or administrative tasks.
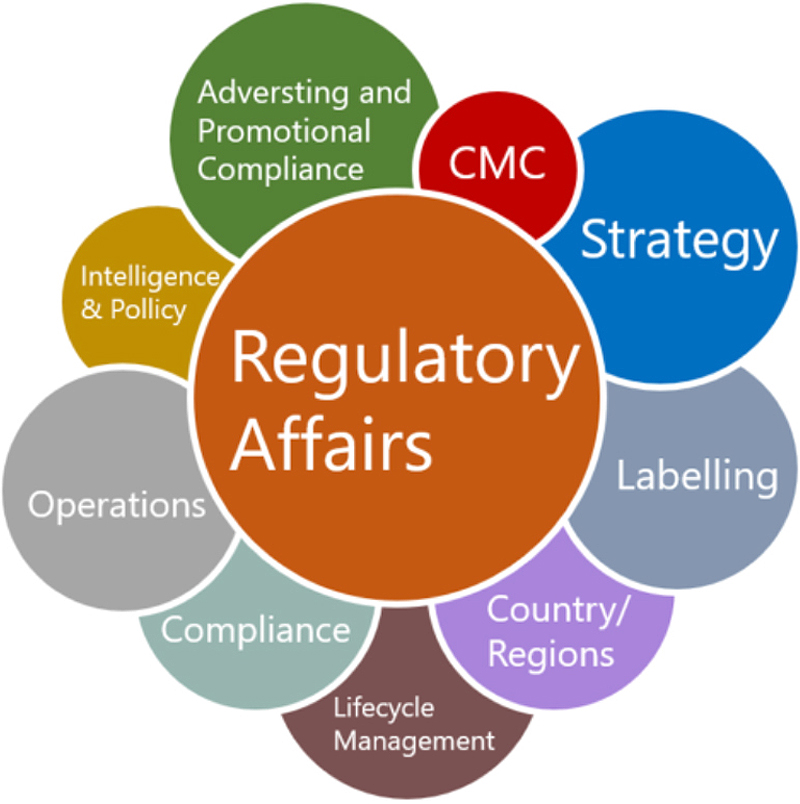
Leading Regulatory Teams to Deliver Strategic Value to Modern R&D
Regulatory affairs remain an area with several different subfunctions that benefit from such diversity, such as strategy, operations, CMC, intelligence and policy, advertising and promotional compliance, and labelling, and all these subfunctions require professionals with different backgrounds. Regulatory affairs leaders need to embrace the “big tent” mentality and create environments where both pluralists and specialists can fully realize their potential. In any case, leaders need to be clear in setting roles and responsibilities across the organization so their teams can operate comfortably and find appropriate development pathways.
Other than that, the current context has highlighted the need for regulatory leaders to be mindful of the work-life balance traps of working remotely. Regulatory professionals work almost entirely in front of a laptop or in meeting rooms with other teams, and it is important in a time of confinement to respect workload and personal life balance. This is particularly important in companies with an extensive global footprint, where teams with members from all over the world need to connect at the same time, which can be incompatible with people’s schedules. Supporting staff during this time remains an important commitment.

