The Value and Funding of Alzheimer’s Disease Treatments
A Multi-Stakeholder Initiative
Nicholas Gertler
Co-Founder
Matt Diver
Co-Founder
lzheimer’s disease presents an enormous global burden. Despite historic setbacks, significant research and development resources continue to pour into the project of finding an effective treatment. But developing a drug that works is only part of the challenge. Health systems and innovators need to be ready to deal with the unique challenges that the introduction of these drugs will present. Given the complexity of the task and the level of popular and political interest in the outcome, healthcare systems are well-served to plan ahead or face rushed decision making amidst significant public pressure once an efficacious drug is reported.
Preparing for Scientific Success in the Development of Alzheimer’s Disease Treatments
Pathways for the Introduction of Alzheimer’s disease Treatments (PIAdT) is a multi-stakeholder initiative designed to help prepare European health systems for scientific success in the development of Alzheimer’s medicines. PIAdT will address how to properly assess, fund, take up, and use first-generation Alzheimer’s disease treatments. Participants in PIAdT include pharmaceutical companies advancing Alzheimer’s disease (AD) pipelines; government officials charged with assessing and funding these medicines, clinicians and health system leaders caring for patients and managing the uptake of new treatments, and foundations advancing the cause of research and patient care. Galen/Atlantica is designing and chairing PIAdT in partnership with Alzheimer’s Research UK. The initiative is launching in late fall of 2018, with a work program that will begin to report results in 2019.
The Challenge Facing the Introduction of an Emerging Alzheimer’s Medicine
As the only top 10 cause of death that cannot be cured, prevented, or slowed, AD presents an enormous global burden. AD is the most common cause of dementia, contributing to an estimated 60-70% of all dementia cases. According to the WHO, around 47.5 million people live with dementia worldwide. By 2050, this number is expected to exceed 130 million. Within this timeframe, the total cost of AD, driven primarily by social care and informal care, will be on the order of $1 trillion.
Alzheimer’s presents a significant unmet medical need that has motivated significant investment in developing a treatment to change the course of the disease. This investment, however, has thus far yielded little in the way of results. It is a widely-cited statistic that, over the past 15 years, 99.6% of Alzheimer’s drug candidates have failed to show efficacy. The exception, meantime, provides a temporary boost to cognitive function without altering the course of the disease.
These setbacks have yielded an ongoing process of learning, and industry continues to advance pipelines of potentially disease-modifying treatments (DMTs). Possibly as soon as 2020, one or more of these medicines will be shown to be efficacious.
The emergence of an AD DMT will expose a significant tension: there will be enormous public pressure on all healthcare stakeholders to deliver the DMT to patients. However, the medicines ecosystem is at risk of being unprepared to properly assess, fund, take up, and use such a medicine, and identify patients who will benefit. Specifically, value assessment methodologies do not have a ready way to translate the clinical trial data accompanying a DMT into a measure of long-term benefit. Additionally, the introduction of a DMT will place an immediate and significant demand for funding on national drug budgets that will not be offset by direct, near-term cost savings.
The Opportunity for Multi-Stakeholder Engagement to Address these Challenges
Given the complexity of these challenges and the variety of adjustments needed across the medicines lifecycle, no single company or institution can succeed by acting alone. AD developers and public agencies, informed by clinicians and patient voices, need to engage jointly in the design and piloting of appropriate value recognition and funding models for first-generation AD DMTs. This recognition led to the creation of PIAdT.
PIAdT is a pre-competitive initiative supported by the major companies of the pharmaceutical industry who are developing AD DMTs. Alzheimer’s Research UK, the UK’s leading AD research charity, is acting as co-convener. PIAdT has been fully recruited, with nearly 30 participants representing European regulators, HTAs, payers, ministries of health, clinical and academic experts, and patient representatives having committed to working together to address the challenges of AD value assessment and funding (Figure 1).
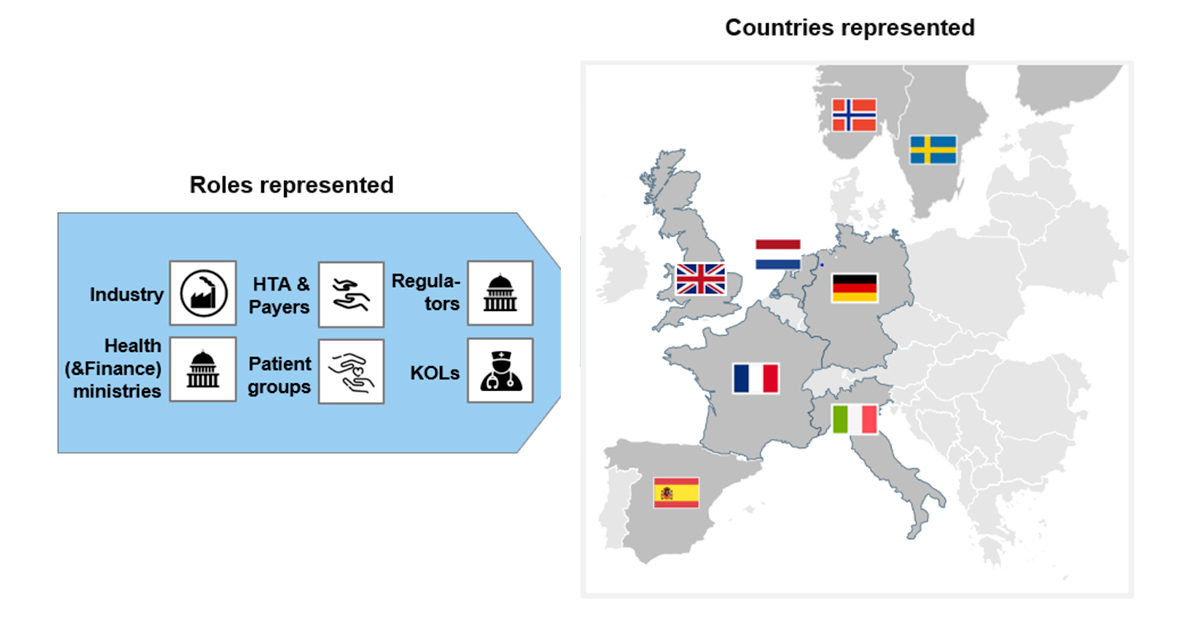
Figure 1. PIAdT participants span multiple countries and health system roles.
Specific Value Assessment and Funding Challenges to be Addressed
Our discussions with PIAdT participants from across the spectrum of health system constituencies has brought into focus the specific challenges to value assessment and funding for the first generation of Alzheimer’s treatments. These issues are listed in Figure 2, and we briefly address each of these issues below.
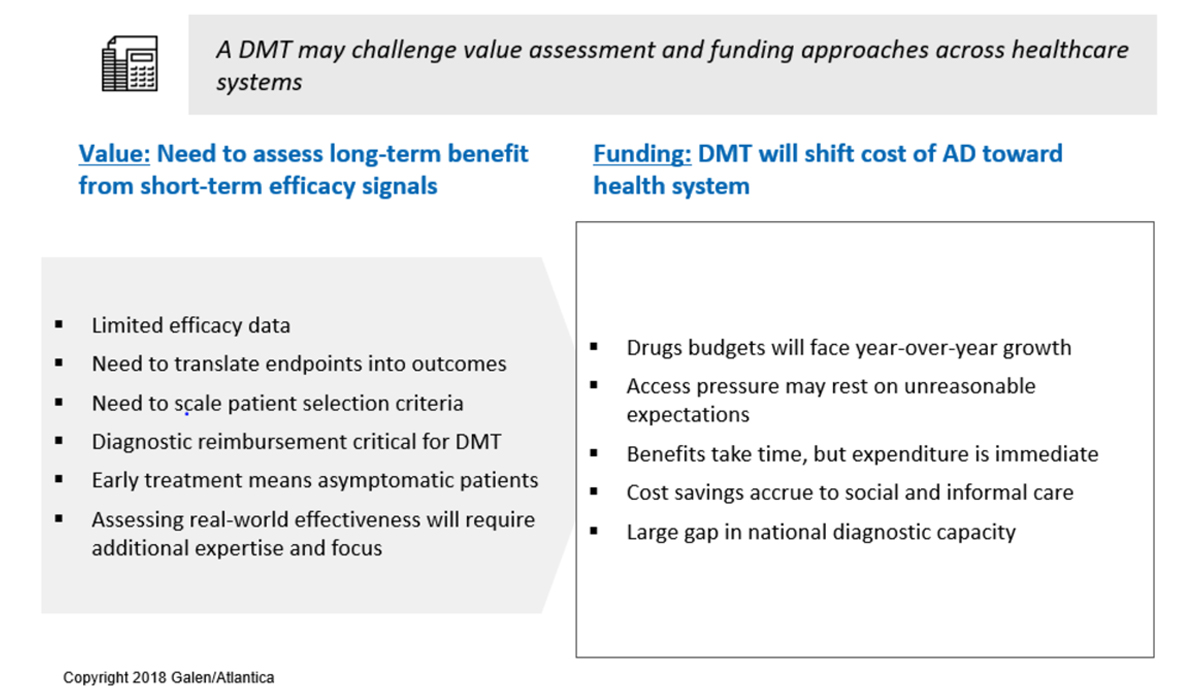
Figure 2. Value assessment and funding issues facing the next generation of AD treatments.
Challenges for Value Assessment
- Limited efficacy data – Randomized Clinical Trials (RCTs) follow patients for only 18-36 months, while the cognitive decline precipitated by Alzheimer’s plays out over decades. Value assessment methodologies will need an approach for drawing conclusions about long-term effectiveness from data on, comparatively, short-term efficacy.
- Need to translate endpoints into outcomes – In order to be manageable, RCTs in AD measure effect on a set of cognitive and/or functional scales, with efficacy represented by a divergence in progression on these scales between treatment and control arms. Work remains to be done to demonstrate that the measured differences translate into meaningful and sustained changes in health and functional status.
- Need to scale patient selection criteria – RCTs select patients utilizing accurate but costly diagnostic and monitoring techniques, such as PET scan and MRI, that face cost and capacity constraints if applied at population scale.
- Diagnostic reimbursement is critical for a DMT – The patient journey in AD will need to pass through a diagnostic funnel that starts with low-cost screening and passes through confirmatory diagnostics. Without reimbursement of these procedures, which may include PET imaging or cerebrospinal fluid (CSF) measurements under the current state of the art, patients seeking treatment will face a significant roadblock.
- Early treatment means asymptomatic patients – The evolving understanding of AD etiology increasingly points to early intervention as the key to patient benefit. In practice, this trend will mean treating patients before they exhibit functional impairment due to cognitive decline. Early treatment thereby raises the challenge of convincingly identifying which individuals are on the path to Alzheimer’s before they exhibit the type of clear symptoms to which healthcare systems are designed to respond.
- Assessing real world effectiveness will require additional expertise and focus – Multiple HTA leaders participating in PIAdT have suggested that the ultimate solution to value assessment will not come from developing the “perfect” value model, but through on-going follow-up and learning from the real world use of new treatments. This will require a new set of institutional linkages and infrastructure to be developed.
Challenges to Funding
- Drug budgets will face year-over-year growth – At present, the medicinal treatment of Alzheimer’s is limited to relatively low-cost symptomatic treatments introduced several years ago. The introduction of a new AD drug will place a significant new demand on drug budgets. And because so much of the cost of AD is born outside of the healthcare system, there will be no short-term savings to offset the cost of these drugs.
- Access pressure may rest on unreasonable assumptions – In the words of one public agency participant in PIAdT, “[T]he scientific logic from the clinical trials, including who to treat, will fall apart once newspaper headlines shout of an ‘AD breakthrough’.”
- Benefits take time, but expenditures are immediate – The current expectation regarding AD treatments in the pipeline is that they will slow the rate of decline, but not restore cognitive capacity already lost to Alzheimer’s. Healthcare systems will therefore be asked to pay now to reduce disease severity several years in the future.
- Cost savings accrue to social and informal care – These sectors lie outside the traditional budget silos of the healthcare system, making cross-comparisons and reallocation difficult. In addition, health system leaders caution that attributing savings in social and informal care to medicinal interventions will be analytically challenging.
- Large gap in national diagnostic capacity – The patient journey will require ensuring adequate national diagnostic capacity to be in place, which includes capital equipment in addition to the cost of specialists and individual tests.
Pathways to Progress
These challenges provide fertile ground for thoughtful engagement by a multi-stakeholder group. PIAdT is designed to deliver concrete, actionable approaches to value and funding for a generation of new Alzheimer’s treatments. To support these outcomes, a key role for the conveners is to introduce the tools and structure for collaborative problem-solving. Supported by this armamentarium, PIAdT participants are committed to progress in preparing healthcare systems for the long awaited time when clinical trial readouts will signal the dawn of efficacious treatments to slow the march of this terrible disease.

