Bayer AG
Lundbeck
EFPIA HTA Working Group
nly a few months remain before developers must follow a new market access route for new cancer products and advanced therapy medicinal products (ATMPs) in the European Union (EU). Traditionally, health technology assessments (HTAs)–evaluations of the clinical effectiveness and value of new medicines and devices–were conducted by individual EU member states. This approach led to redundancies and potential delays in bringing new treatments to patients, particularly on clinical benefit assessment. The EU HTA Regulation and the introduction of Joint Clinical Assessments (JCAs) aim to streamline these evaluations.
As the process takes shape many questions remain: How will the EU- wide assessment of clinical evidence by HTA bodies work in parallel with the Marketing Authorisation review by the European Medicines Agency? How can industry and government bodies best prepare? This article dives deeper into the learnings from a DIA Europe 2024 HTA Townhall discussion of this topic and explores what steps still need to be taken to make it work.
The DIA Europe 2024 HTA Townhall brought together a broad spectrum of key players on the HTA stage: Maya Matthews, European Commission; Niklas Hedberg, TLV, HTA CG Co-Chair Medicinal Products; Paul De Boissieu, HAS, Chair HTA CG JCA Subgroup; Michael Berntgen, EMA; Valentina Strammiello, European Patients’ Forum; Isabelle Stoeckert, Bayer AG; and Inka Heikkinen, EFPIA HTA Working Group (Lundbeck).
(All poll results courtesy of DIA.)
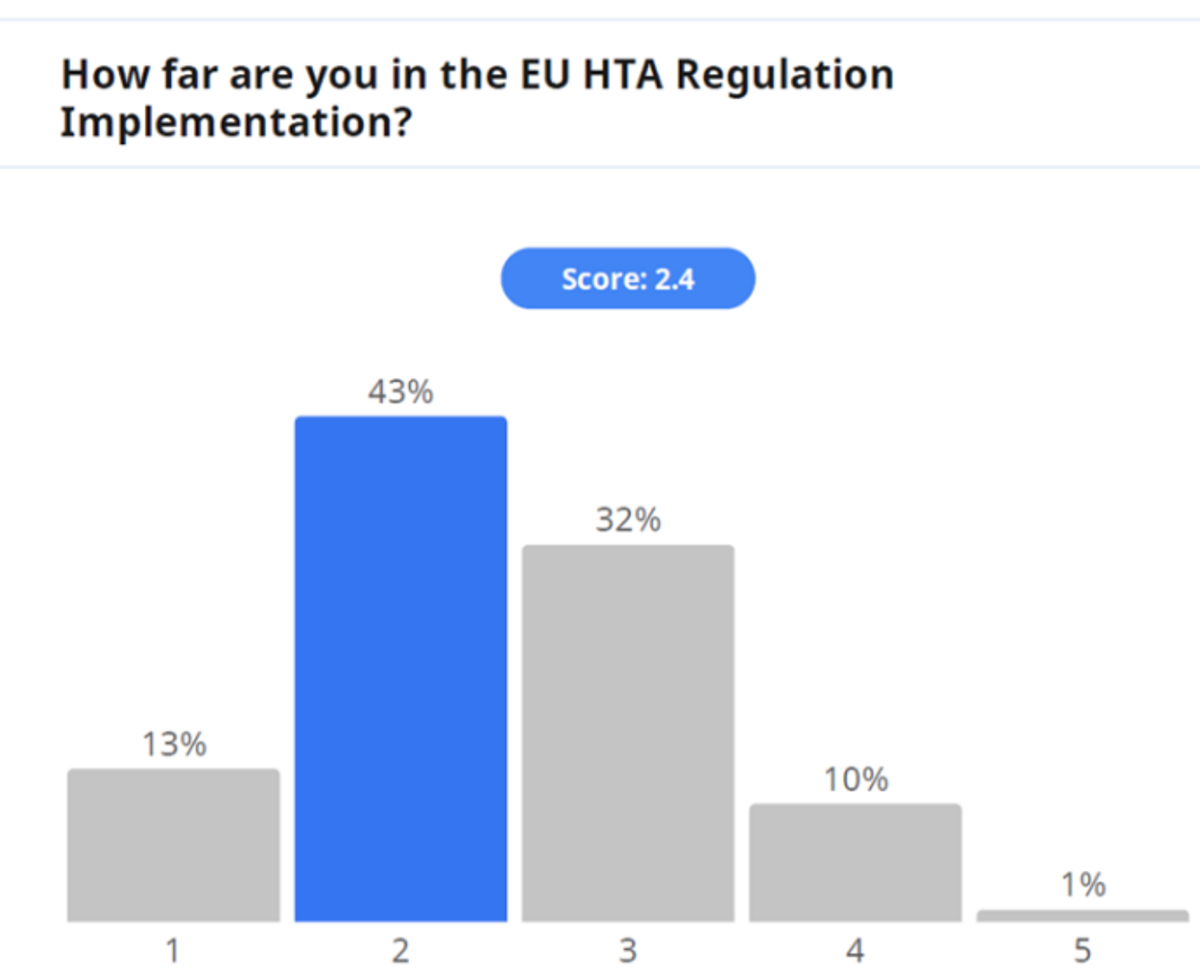
The implementation of Joint Clinical Assessments in January 2025, starting with new cancer products and ATMPs, necessitates adjustments on the part of all stakeholders. In particular, the HTA timeline becomes a key factor because assessments are due to start after validation of the Marketing Authorisation Application (MAA) submission. National HTA bodies will have a designated period to establish the assessment scope (Determination of the Population, Intervention, Comparator, and Outcome [PICO]) followed by a sponsor-designated timeframe for submitting the JCA dossier. This compressed timeline underscores the importance of well-defined internal procedures for a smooth transition.
Paul De Boissieu (Chair, JCA Subgroup of HTA Coordination) emphasized the intention of the JCA to support the member states in their national assessments. The main objectives include (1) to not repeat (request) the analyses at the national level; (2) to draw conclusions (on reimbursement, added value, and related topics) at the national level; and (3) to use high-quality JCA to provide faster access to medicinal products and medical devices.
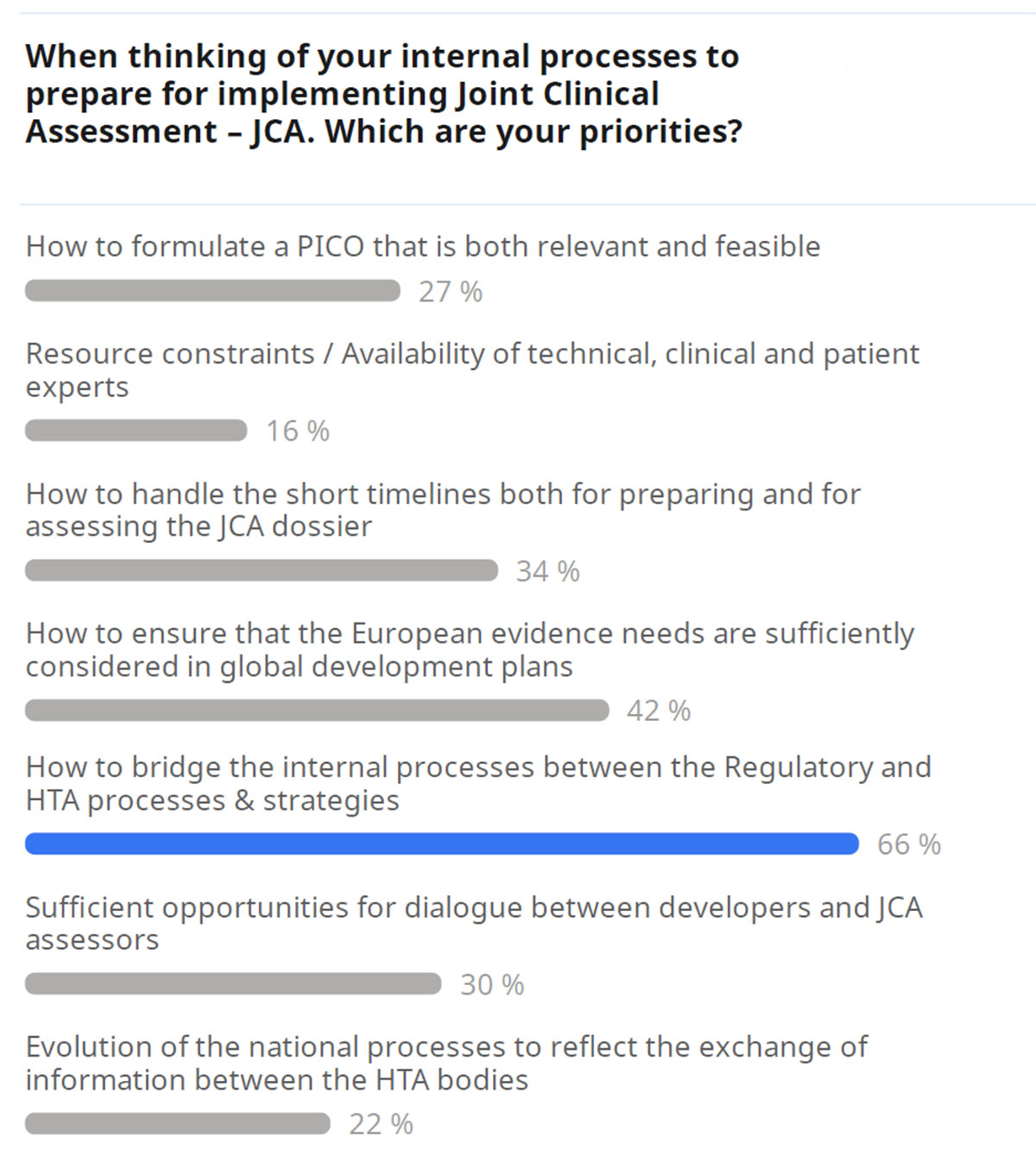
These considerations highlight the importance of thorough preparation for the JCA process. While challenges are anticipated, proactive planning can help ensure a smoother transition and successful navigation of the new assessment procedures. Polling the audience (Figure 2) revealed the top internal process priorities from the perspective of industry.
The potential for a “learning HTA system” across the EU is a noteworthy opportunity. This includes transparency on the available data and sharing of best practices, with patient interests in mind. If national HTA processes adapt effectively and resources are strategically shifted towards the EU level, the overall system could become significantly more efficient. This, in turn, could lead to faster access to innovative healthcare technologies for European patients.
The upcoming Joint Clinical Assessment also holds opportunities for progress. The focus on innovative therapies, such as precision medicine and Advanced Therapy Medicinal Products (ATMPs), may necessitate the adoption of new scientific standards for assessment. Methodologies may need to evolve with the emergence of such new technologies, and the new structure under the EU HTA CG could provide a forum for mutual learning.
Joint Clinical Assessment introduces a novel streamlined process for HTA evaluations. Industry needs to be prepared to adapt internal processes and effectively manage dossiers within the designated timelines.
To ensure a smooth transition to the JCA process, industry efforts should prioritize several key actions. Figure 3 shows the audience poll results on priority industry tasks to prepare for JCA:

Collaborative PICO Scoping: Industry should explore opportunities to leverage the expertise of multiple countries during the scoping phase. This collaboration can help establish a more comprehensive understanding of potential assessment scope.
Integrated Evidence Strategy: Developing an early and integrated evidence generation plan and incorporating Joint Scientific Consultations (JSCs) where applicable, will be essential for preparing the required evidence base and proposed comparators. It may help to prepare for the subpopulations or subgroups, and respective comparators, that could be requested in addition to the full population, which is different from the usual regulatory process.
Close Team Alignment: Maintaining close collaboration between EU market access and regulatory teams is paramount. This will help avoid duplication of effort and ensure a cohesive approach to navigating both regulatory and HTA requirements.
Case Study: How One Multinational Pharmaceutical Company Prepared for EU HTA Regulation
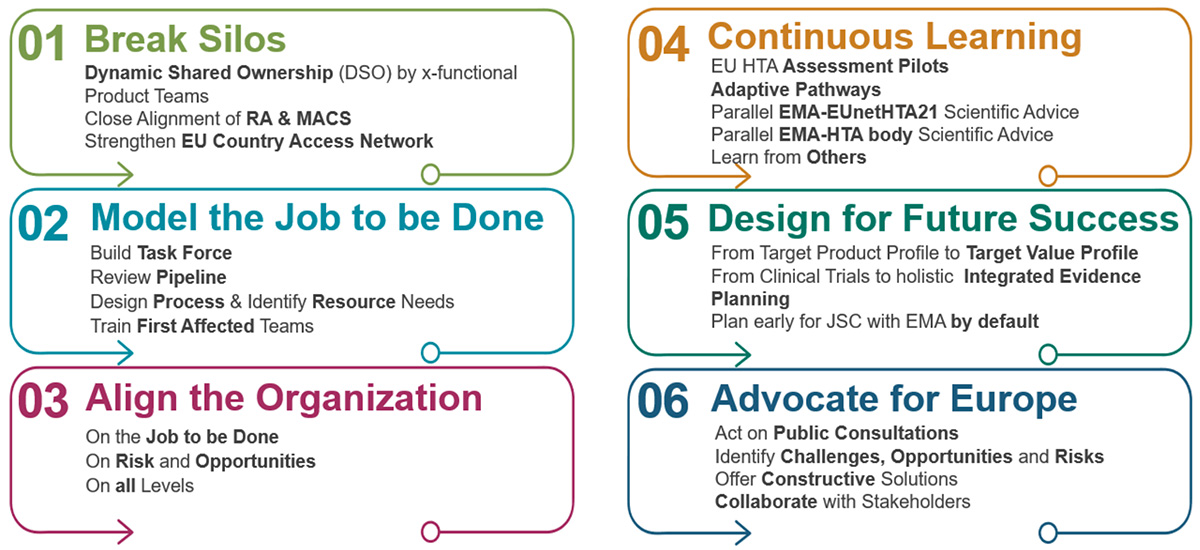
1. Breaking Silos: Foster an agile culture with closer collaboration between Market Access and other departments. Additionally, Market Access colleagues are actively building strong networks with their EU counterparts, mirroring the established collaboration between the regulatory team and EU regulatory authorities.
2. Modeling the Job to be Done: The company has identified its most likely “first affected product” and predicted potential PICOs associated with it. Based on this model, the necessary processes and resources to handle the JCA workload are being defined. While this may require additional work upfront, it allows for anticipating challenges and developing effective solutions in response. In this case, the company will have to leverage the strong expertise of the national EU affiliate access teams to ensure a comprehensive understanding of the JCA process.
3. Align the Organization: Prioritize clear communication throughout the organization. This includes informing all pertinent leaders of the organization and all potentially impacted teams about the upcoming JCA implementation. Recognizing that transparency across all levels is essential for a successful transition, this preparation actively fosters open communication channels.
In addition to these core steps, the company is also leveraging its previous experiences (see below) to further enhance its JCA preparedness.
4. Continuous Learning: The company has actively participated in various pilot programs over the past decade. This includes involvement in EU HTAs, adaptive pathways, parallel HTA EMA advice, and other initiatives. These real-world experiences provide valuable insights and allow to “pilot and drive learning” within the JCA framework.
5. Design for Future Success: The company established clear goals for successful JCA implementation. One core principle is demonstrating a strong “True Value Proposition” (TVP) for patients, believing it to be the foundation for positive JCA appraisals by healthcare systems. We advocate for early and comprehensive integrated evidence planning. This strategy explores how real-world evidence (RWE) can be strategically combined with traditional clinical development plans, which is particularly important for Advanced Therapy Medicinal Products (ATMPs) and oncology products. To ensure preparedness for PICO scoping discussions, teams are encouraged to routinely plan for Joint Scientific Consultations (JSCs) with the EMA. This proactive approach equips them with valuable insights that can inform the JCA process.
6. Advocate for Europe: Recognizing the current industry trend of prioritizing the US market, the company is actively advocating through its policy teams for a more competitive EU regulatory innovation framework. The belief is that a robust and efficient European regulatory framework will ultimately benefit all stakeholders involved.
While the specific details of this one multinational pharmaceutical and biotechnology company approach may not translate directly to smaller companies, the first three points–breaking down silos, modeling for success, and clear communication–are more universally applicable.
Plan, Allocate, Anticipate
National HTA bodies, particularly smaller agencies, may face resource constraints. Effective planning and allocation of resources will be essential for timely and efficient JCA implementation. Valentina Strammiello (European Patients’ Forum) stated how important it is to keep the processes as transparent as possible and to anticipate as much as we can. She would like to see as “a commitment from member states to deliver on the implementation of the regulation, because that’s the main reason why we are doing it: Avoid duplication of effort, providing faster access to medicinal products and medical devices. Make sure that also the quality of joint clinical assessment is ensured. This is also meant to support those member states that do not have very mature HTA systems yet. That’s where I see really the major benefits in the first years of the implementation.”
Policymakers can play a crucial role in supporting industry preparation for JCAs through several key measures (Figure 5 shows audience polling results about this role):

Streamlined PICO Consolidation: Developing a clear methodology for consolidating PICOs across different national HTA bodies would be highly beneficial. This would provide industry with a more comprehensive and consistent understanding of potential assessment scope.
Transparent Interaction Guidelines: Establishing pragmatic rules for stakeholder interaction within the framework of the Implementing Act would be valuable. Reflecting on the nature, timing, and content of interactions with HTA decision-making bodies can provide clarity for all parties involved and ensure a smoother JCA process.
The coming months will be crucial as EU member states and stakeholders navigate the implementation of the HTA Regulation and JCAs. The success of these measures will depend on ensuring a smooth transition, clear communication, and ongoing collaboration between all involved parties. “The commitment by the panel is clearly there. We need to collectively establish the most suitable practices for this interface,” Michael Berntgen (EMA) summarized.
By proactively preparing and working together, industry and policymakers can ensure a successful rollout of JCAs, ultimately leading to fulfill the initial aspiration of the HTA legislation: faster access to innovative healthcare technologies for European patients.

