Parexel
arnessing the strengths of spatial biomarkers offered by digital pathology and spatial genomics can give researchers deeper understanding of disease complexity, help pinpoint new therapeutic targets, and forge the path towards more precise and efficacious therapies. These transformative technologies have the potential to revolutionize precision medicine and accelerate the development of novel therapies for various diseases. As a result, major stakeholders, including large biopharmaceutical companies, research institutions, genomics laboratories, diagnostic developers, and data analytics and AI companies, are increasingly focusing their attention and investments on these new frontiers.
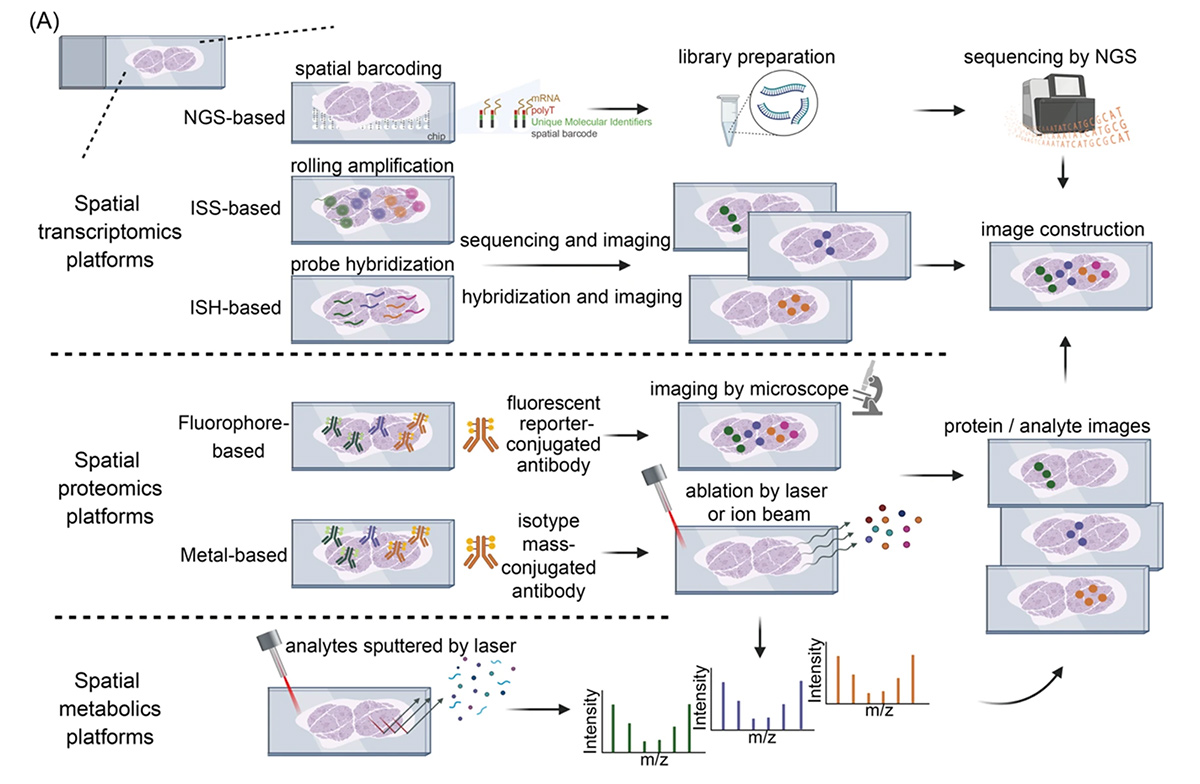
Digital Pathology and Spatial Genomics Provide Enriched Biomarker Information
AI in Digital Spatial Biomarkers
- Image analysis and pattern recognition: AI/ML/DL algorithms are utilized for analyzing large-scale digital images obtained from techniques such as spatial transcriptomics, IHC (immunohistochemistry), fluorescence in situ hybridization (FISH), or multiplexed biomarker imaging. These methods involve segmenting cells, identifying nuclei, and quantifying spatial patterns of gene expression. For example, convolutional neural networks (CNNs) are being employed for image segmentation, object detection, and precise analysis and quantification of specific cellular or molecular features within the normal and tumor tissues.
- Spatial clustering and automated classification: AI/ML/DL algorithms can be deployed for supervised or unsupervised learning tasks to detect spatial cell states, quantify molecular biomarkers, classify different immune cell subgroups, and characterize complex tumor microenvironment.
- Spatial biomarker data Integration: AI/ML/DL techniques enable seamless integration of spatial biomarker data with diverse “-omics” data modalities, including spatial proteomics and single-cell RNA next-generation sequencing (NGS) data. This integration encompasses both spatial and temporal dimensions, thereby augmenting the richness and comprehensiveness of the combined data set and leading to enhanced biomarker insights and value extraction.
- Predictive and prognostic modeling: AI/ML tools are particularly transformative in leveraging large data sets to develop prognostic and predictive modeling in areas such as precision oncology. By digitalizing and analyzing multimodal histopathological images along with multi-omics data (e.g., genomics, proteomics, molecular pathology), clinical data, and real-world data, these models can predict disease progression, treatment responses, and patient outcomes for precision medicine development and clinical decision-making.
Promises of Spatial Biomarkers
- Drug target identification: Spatial “-omics” profiling can reveal the spatial distribution and expression patterns of target genes or proteins within tissues. Such information can help create molecularly defined tissue atlases within cellular resolution and guide development of therapeutics targeting specific cellular populations or microenvironments.
- Novel biomarker discovery: Digital pathology and spatial genomics enable identification and validation of novel biomarkers associated with diseases. By correlating molecular profiles with tissue morphology, biomarkers with improved predictive value can be identified for improved diagnosis or prognosis.
- Disease mechanism elucidation: The combination of molecular profiling and tissue architecture analysis helps unravel complex disease mechanisms and cellular interactions. This understanding can lead to earlier or improved disease diagnosis, and discovery of new pathways and targets for therapeutic intervention.
- Precision diagnosis and personalized therapy: The integration of molecular or histological features with spatial context and AI/ML/DL facilitates precision diagnosis of specific diseases or subtypes, enabling more accurate patient stratification, optimizing precision clinical trial design, and ultimately personalized treatment decisions.
- Treatment response prediction and monitoring: Digital pathology and spatial genomics can provide real-time monitoring of treatment responses at both cellular and molecular levels. This allows for the assessment of therapy efficacy and the identification of responder versus nonresponder and potential resistance mechanisms.
What Are the Challenges to Realizing This Promise?
- Computational complexity and scalability: Analyzing spatial biomarker data requires sophisticated computational algorithms and infrastructure. The analysis of large-scale data sets can be computationally intensive and time-consuming, requiring substantial computing resources. Developing efficient and scalable AI/ML/DL algorithms that can handle the complexity of spatial biomarker data will continue to remain crucial.
- Analytical and clinical validation: Translating promising findings from spatial biomarker and AI research into clinical applications demands both rigorous technical and clinical validation (often requiring clinical trials). Sufficiently demonstrating the clinical utility, reliability, and reproducibility of spatial biomarkers and AI algorithms in the target patient populations and intended purpose use contexts is challenging yet essential for their widespread adoption in drug development and clinical applications.
- Cost and access to resources: Spatial biomarker analysis often necessitates access to integrated high-quality data sets, high-resolution imaging technologies, high-performance computing infrastructure, advanced AI-based algorithms, and interdisciplinary expertise. However, the cost and accessibility of these resources can pose challenges for researchers, especially those in resource-limited settings.
- Regulatory considerations: Obtaining regulatory approval for AI-driven spatial biomarkers or diagnostics is needed for their applications in drug development or clinical settings. This calls for well-defined regulatory guidelines and frameworks that account for the distinct characteristics of spatial biomarkers and AI algorithms. Promisingly, FDA is actively involved in regulating and overseeing the use of AI/ML in diagnostic and drug development and has taken proactive measures such as releasing a discussion paper to gather feedback from stakeholders to address the challenges involved and establish a flexible framework that fosters innovation.
Looking Forward
Captain James Kirk: “Space, the final frontier… Its continuing mission: to explore strange new worlds; to seek out new life and new civilizations; to boldly go where no one has gone before!”

