Research Involvement and Engagement, BMC
atient authorship of peer-reviewed publications is increasing, with patients not only presenting their own or academic research, but also participating as co-authors of pharmaceutical company-sponsored research. In this two-part series we:
- highlight the drivers and value of involving patients as authors from both the patient and industry perspectives (Part A), and
- discuss how journals are evolving to facilitate inclusion of the patient voice in peer-reviewed publications and extend the reach of publications to lay audiences (Part B).
Drivers, Opportunities, and Barriers for Patient Authors
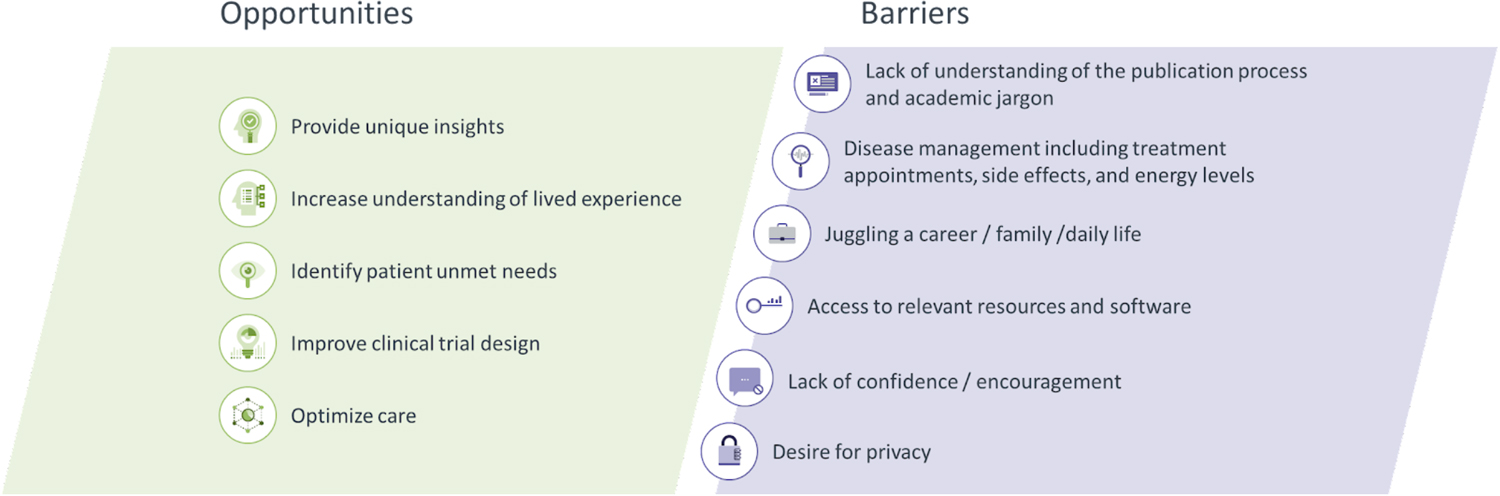
However, not all patients can or wish to author peer-reviewed research publications. To be successful, patient co-authors may have to overcome several barriers (Figure 1). Often, the greatest barrier is a lack of familiarity with the peer-review process and academic language used in research publications. Managing illness and treatment side effects and maintaining energy levels may also present hurdles that are difficult to overcome. Other barriers are more societal and include juggling job demands or family commitments and accessing technological resources to facilitate collaboration or attend online meetings. Such barriers can result in a lack of confidence to co-author articles alongside experienced academics. Furthermore, some patients may not wish to be identified for privacy reasons and to avoid any stigma that may be associated with their condition.
Optimal Co-Authoring Between Pharmaceutical Companies and Patient Partners
Indeed, one company recently established a Patient Collaborative Board, whose objectives include enhancing partnerships with patients in the development of scientific publications. A key output of this group is enterprise-wide patient authorship guidance, designed to enable patient co-authorship of publications across the company. The guidance includes potential author identification, contracting, collaborating, and reimbursement. Training for employees on how to implement the guidance has also been developed.
A recent publication, led by two patient authors and supported by another company, demonstrates the value of patient insights to better understand their lived experience, especially in rare diseases. Such studies can teach us about how industry can best partner with patients in research and publications. Key learnings for industry from this study, in which two of the authors of this article were involved (DL and VP), include the following:
- Liaise closely with internal compliance teams from the outset and throughout the study.
- Collaborate with patient partners who have relevant insights, interest, and time to invest in the study, and the confidence to express opinions.
- Schedule sufficient time to allow patient partners to manage other commitments in their lives.
- For all research involving human participants, ensure that all relevant consent and ethics approvals are in place at the outset of the study, including signed ICMJE forms that confirm the proper reporting, and ethical preparation of research manuscripts submitted to peer-reviewed biomedical journals.
- Access relevant resources and training to facilitate academic, company, and patient author collaborations.
Companies are also supporting the development of narrative reviews. Such reviews examine the current understanding of specific conditions and how best to improve patient outcomes by leveraging clinical practice, scientific literature, and the experience of patient authors, as illustrated in this example.
Evidence-Based Training and Resources to Enable Patient Authorship

SUPPLEMENTAL RESOURCES
Hidden in plain sight? Identifying patient-authored publications
ICJME: Defining the role of authors and contributors
WECAN training module on “Patients in Publications”
The lived experience of myasthenia gravis: a patient-led analysis
Guidance on authorship with and acknowledgement of patient partners in patient-oriented research
Taking patient involvement in clinical publications to new heights (Presented at Patients as Partners Europe, 2022, Sykes & Bharadia)

