Around the Globe
Part 1: Introduction and Strategic Frameworks
ver the past five years, China’s pharmaceutical innovation industry has experienced what has been called the Cambrian explosion: significant acceleration in the launch of innovative products, rapid expansion in the number of R&D pipelines, and increasing capital investment in R&D.
To help address this gap, DIA China’s R&D Talent and Capacity Building working group launched the China Pharmaceutical R&D Talent Innovation Research Project, which received wide support and participation from many leaders in China’s innovative pharmaceutical industry, in early 2021.
As a global organization whose mission includes developing R&D talent and capability, DIA is committed to internationalization through innovation. This includes linking China’s R&D talents through the DIA platform to ultimately develop industry leadership that will establish a more dynamic R&D talent ecosystem in China.
This project team conducted in-depth interviews with both R&D and human resources (HR) leadership in the pharmaceutical industry. By observing and analyzing the different challenges these leaders currently face, this two-part article aims to promote increased cooperation among global pharmaceutical R&D talent and in particular to increase awareness of new talent strategies, and to strengthen global talent innovation in China. It also aims to promote aligning the education system with standard clinical research competency requirements to lay a solid foundation for future Chinese pharmaceutical industry talent.
As we prepare for the next five years, we must ensure that the scientific, technological, and talent challenges to innovative R&D do not hinder the sustainable growth of China’s innovative pharmaceutical industry.
Strategic Thinking and Leadership Improvement
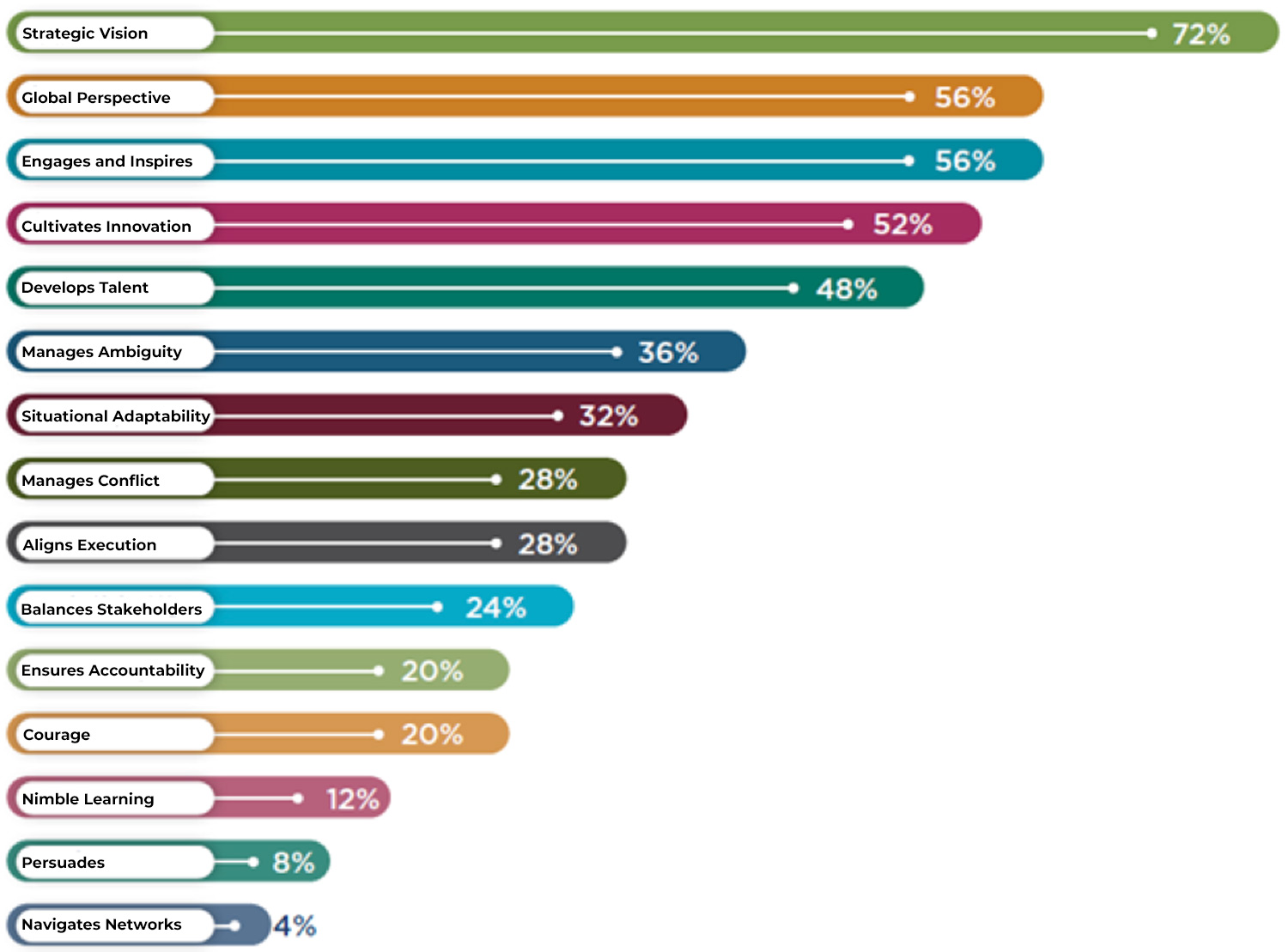
Figure 1: Key dimensions of R&D organizational leadership in pharmaceutical companies that need improvement by percent of responders.
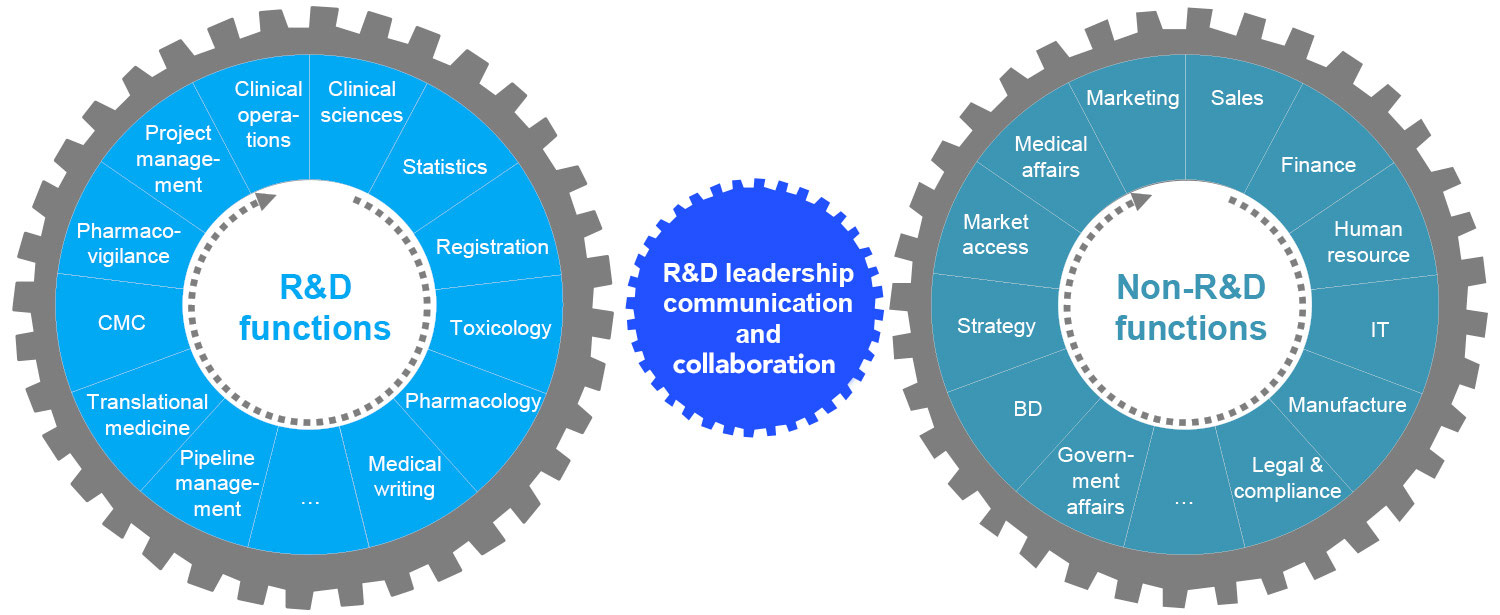
Stable backbone
Dynamic capabilities
Robust processes to support core R&D work
Leaders and teams can focus their time and energy on innovative ideas and other value-creating work.
Leaders and teams have the ability to rapidly tap into data and technology opportunities (e.g., companion diagnostics, advanced analytics for site selection).

