Magnolia Place Consulting
heckpoint inhibitor therapy with ipilumumab, nivolumab, pembrolizumab, or similar antibodies has produced outstanding clinical responses in some patients with advanced carcinomas or melanomas. However, most patients are resistant to checkpoint inhibitors because their immune responses are blocked by an immunosuppressive tumor microenvironment (TME). Resistance is characterized by a lack of immune cells infiltrating tumors, stromal macrophages and fibroblasts that release pro-metastatic cytokines, and immune cells that are exhausted and dysfunctional. To address this resistance, we focus on a process called immunogenic cell death (ICD) that rejuvenates antitumor immunity and changes the TME to an antitumor state.
ICD is a pre-death state induced by select therapeutic agents (Table 1) as they activate regulated cell death programs that include, but are not limited to, apoptosis, necroptosis, ferroptosis, or pyroptosis. ICD is characterized by the externalization of calreticulin (CALR), an endoplasmic reticulum chaperone involved in calcium homeostasis and protein-folding quality control, on the outside of the plasma membrane. Ecto-CALR occurs before the externalization of phosphatidyl serine in cells undergoing apoptosis and acts as an “Eat Me” signal attracting lymphocytes, dendritic cells, and other antigen-presenting cells (Figure 1). As CALR reaches the cell surface, intracellular ATP and other damage-associated molecular patterns (DAMPs) such as high mobility group box 1 (HMGB1) and heat shock proteins 70 or 90 kDa (HSP70/90) chaperones are released to initiate the innate and then adaptive immune response.
HMGB1 and other chaperones are a “Take Me” signal that simulates the uptake of peptides and neoantigens released from the endoplasmic reticulum into antigen-presenting cells (Figure 1). This pre-death process is accompanied by the release of chemokines, cytokines, and other molecules that activate and mature dendritic cells as they migrate to regional nodes and prime adaptive immune responses to tumor antigens. Not all chemotherapeutic agents are able to induce ICD. For instance, cisplatin does not induce ICD even though it causes apoptotic death, but ICD can be induced if CALR is introduced during cisplatin treatment.
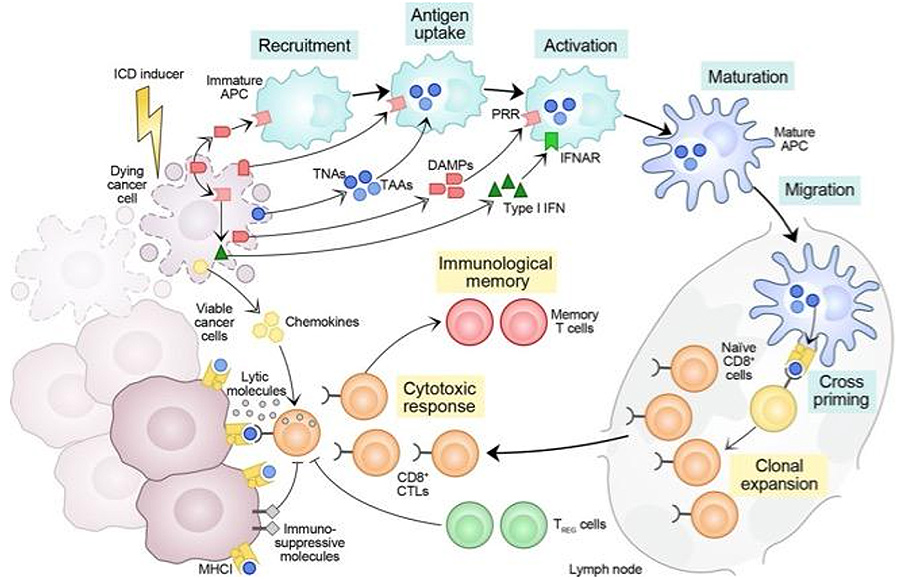
Galluzzi et al. J Immunother Cancer 2020;8:e000337. © Author(s) (or their employer(s)) 2020. Reuse permitted under CC BY. Published by BMJ.
The original instance of ICD was published by Casares and colleagues from the Gustav Roussy Institute in 2005. Murine colorectal carcinoma CT26 cells treated overnight with doxorubicin at more than double the 50% inhibitory concentration (IC50) created cells that vaccinated syngeneic BALB/c immune-competent mice against a challenge with viable untreated CT26 cells. Casares also demonstrated memory and specificity of the antitumor response as well as lack of response to treated CT26 cells in immune-incompetent mice. Subsequent work by this group and others has developed consensus guidelines for evaluation of ICD that have provided broader definition of conditions, effects, and mechanisms as well as how the immune responses are generally restricted to cancer-associated molecules because T cells are tolerized during thymic maturation to self-antigens from normal, nonmalignant cells.
A major problem for clinical ICD is that its inducers must penetrate metastases or primary tumors in sufficient concentration to initiate the pre-death state. The intensity of the ICD induction is critical to creating the pre-death state and may account for the weak abscopal effect that may accompany standard fraction radiation therapy. Standard multidrug chemotherapy or external beam radiotherapy is not likely to cause ICD, especially if the TME is pro-tumorigenic. Oxaliplatin is approved only for systemic administration with either an 85- or 130- mg/M2 infusion dose. However, an infusion of 130 mg/M2 only achieves a plasma concentration of 9 µg/ml or 22.7 µM at 10 min that quickly decreases to 3.7 µM at 50 min. These concentrations are significantly less than the sustained 30-80 µM needed to achieve an effect in vivo. Furthermore, oxaliplatin is rapidly inactivated when exposed to chloride-containing solutions such as plasma or saline. Thus, induction of ICD requires intratumoral injection into either superficial lesions such as led to FDA approval of talimogene laherparepvec or into deep visceral lesions. Interestingly, Europeans have initiated a society to support such therapy: the Human Intratumoral Immunotherapy (HIT-IT) society.
Another problem for clinical ICD is expression of the “Don’t Eat Me” signal CD47 that is overexpressed on tumor cells and binds to signal receptor protein-alpha (SIPRα, CD172a, or SHPS-1) on macrophages and T cells as well as CALR itself to block ecto-CALR initiation of ICD. As a result, CD47 blockers are currently being investigated in preclinical and early clinical trials to convert pro-tumorigenic into antitumor macrophages and to release CALR to initiate ICD.
Finally, we have shown that a single injection of two ICD inducers (oxaliplatin with an oncolytic virus) into large preclinical lesions causes 90% partial responses with 20% complete clinical responses in immune-competent syngeneic mice that also inhibit a viable tumor challenge while increasing T cell infiltration fourfold as well as decreasing CD8+ T cell expression of PD-1 and exhaustion marker. An oncolytic virus has a slower onset of ICD, while oxaliplatin or similar agents have quicker onset of ICD. The combination may increase the time that an injected lesion may function as an antigen depot. Arming virus with a fusogen that causes syncytia formation may further prolong an injected lesion as an antigen depot by stabilizing the pre-death state.
In conclusion, ICD may provide a critical enhancement to checkpoint inhibitor therapy but requires novel approaches to induction that reaches the concentrations needed to activate a stable and prolonged pre-death state combined with blockade of CD47 that stimulates adaptive immunity. However, ICD and CD47 blockade may be a significant tool to overcome resistance to checkpoint inhibitors in many patients.

