Richard Day
University of New South Wales
@osbornidayius
ustralia’s National Medicines Policy (NMP), gazetted in 2000, is being revised and the draft policy was made available for final, widespread community and stakeholder feedback. Final public consultations closed on March 2, 2022.
Outstanding Success
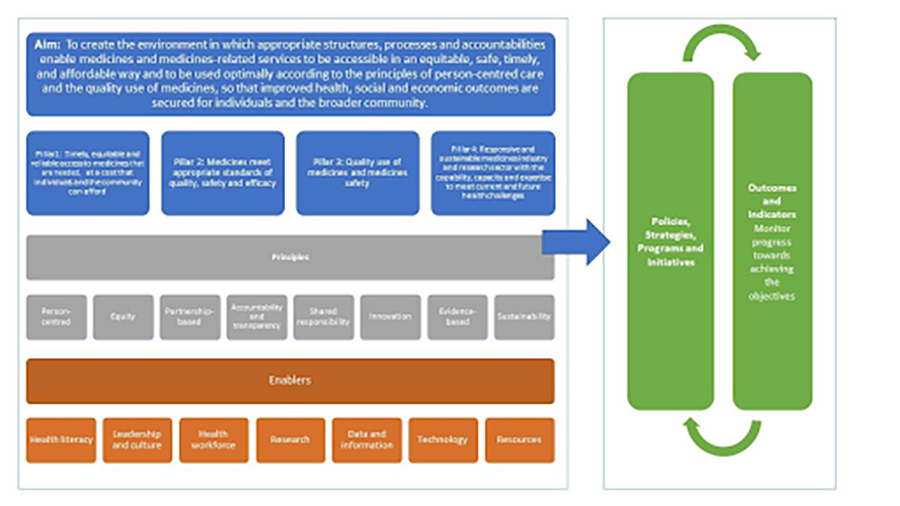
This revision of the NMP, the first after 22 years, was called for by Australia’s Minister for Health, the Hon. Greg Hunt MP, “in recognition of the substantial changes to the health landscape since the policy was published in 2000.” This revision also recognizes the significant contribution of the NMP and its central component of QUM to improving health outcomes for all Australians. The aim of the draft new policy is “to create the environment, in which the appropriate structures, processes and accountabilities enable medicines and medicines-related services to be accessible in an equitable, safe, timely, and affordable way and to be used optimally according to the principles of person-centered care and the quality use of medicines, so that improved health, social and economic outcomes are secured for individuals and the broader community.”
An Expert Advisory Committee chaired by Deputy Chief Medical Officer Michael Kidd AM and supported by Lloyd Sansom AO, Janette Donovan, Sarah Dineen-Griffin, and David Herd (who bring expertise in medicines policy, clinical practice, consumer engagement, and the pharmaceutical industry) is assisting the Minister of Health in revising the Policy. The Committee’s public consultations, bilateral interviews, and virtual group discussion began August 31, 2021, and ended mid-November 2021. A virtual webinar forum discussed key themes from these consultations in December 2021. The 156 submissions, and 194 consultations from 135 organizations, were summarized in a Discussion Paper. The draft “refreshed” policy was then prepared.
Medicines covered by the NMP include prescription, over-the-counter, complementary/traditional, and biologic medicines, gene therapies, cell- and tissue-engineered products, and vaccines. The draft “refreshed” policy consists of four central pillars:
- Timely, equitable, and reliable access to necessary medicines at a cost that individuals and the community can afford: the central role of the widely supported and appreciated Pharmaceutical Benefits Scheme that provides subsidized medicines for all Australians is relevant to this pillar.
- Medicines meet appropriate standards of quality, safety, and efficacy: the well-regarded Therapeutic Goods Administration (TGA) is relevant to this pillar.
- Quality use of medicines and medicines safety: NPS MedicineWise, TGA, and many other entities and organizations are relevant here.
- Responsive and sustainable medicines industry, including nonprescription medicines and research sector with the capability, capacity, and expertise to meet current and future health challenges: increasing numbers of medicines shortages, reliance on ex-Australian suppliers, COVID-19 stresses, etc., are all relevant to this pillar.
The Principles of the proposed refreshed NMP and how they work together have been summarized as person-centered; equitable; partnership-based; accountable and transparent; sharing responsibility; innovative; evidence-based; and sustainable.