Around the Globe
Boehringer Ingelheim
Patient Advocate
nderrepresentation of Black and Indigenous people of color (BIPOC) and other racial and ethnic minorities in clinical trials is a longstanding issue. The disparate impact that COVID-19 has had on BIPOC, Hispanic, Latinx, and Asian communities, combined with the underrepresentation of these subgroups in some COVID-19 clinical trials, has brought a sense of urgency to address this historic challenge. Within the past year, the Pharmaceutical Manufacturers of America (PhRMA) released the first industry-wide principles on clinical trial diversity; the Multi-Regional Clinical Trials Center published the “Achieving Diversity, Inclusion, and Equity in Clinical Research” guidance; and the US FDA released a final guidance to encourage the inclusion of groups that are often overlooked in clinical trials.
The Challenge: Creating More Inclusive Drug Development Process
Creating Solutions: Industry and Patient Collaboration
- Create awareness about the challenges BIPOC, Latinx, and Hispanic patients experience in the healthcare ecosystem.
- Generate ideas and solutions to continue to advance diversity and inclusion across the continuum of clinical development.
Approach: Gathering Diverse Insights, Raising Awareness, and Co-Creating Solutions
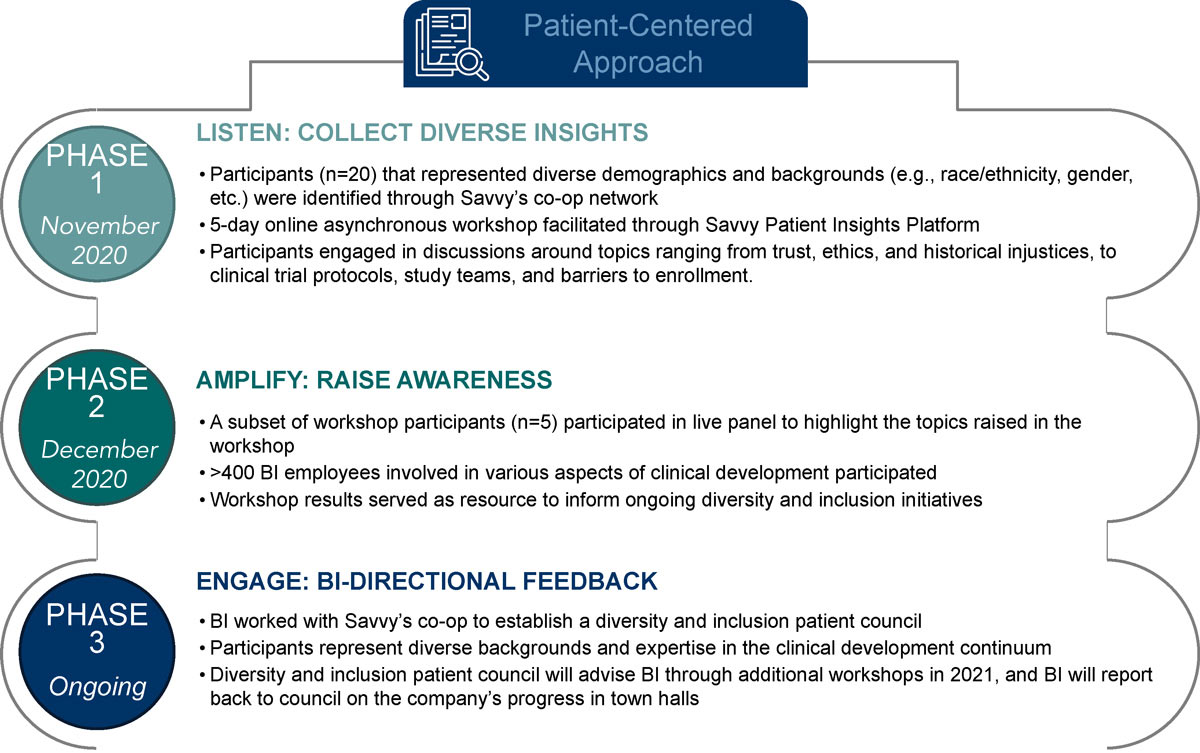
Graphic provided by Boehringer Ingelheim.
Recommendation: Listen and Learn
There is a human tendency to jump directly to solution-oriented thinking. In truth, there is no easy fix to address intergenerational trauma endured by many communities of color. The first step is simply to listen.
“My tribe was recently invited to be a part of a clinical trial for a COVID vaccine but too many people were concerned about safety and questions weren’t being answered so the tribe decided not to participate.”
Recommendation: Building Trust
Recommendation: Build Relationships with Communities
This may require thinking beyond well-established national organizations, to working with grassroots and local community-based groups in order to be inclusive of those not traditionally represented by large organizations. Dialogue with diverse patients should start early on in order to identify unmet needs and provide ample opportunities to work together.
Recommendation: Co-Design Solutions
Workshop participants were asked about the importance of including diverse patient perspectives in the design of a clinical trial, and 100% indicated this is important. 80% said that knowing diverse patients informed the design of a trial would impact their decision to participate. Co-designing solutions across the clinical trial continuum with diverse, representative patients will help ensure that the needs and preferences of all patient communities are considered.
Being mindful of power dynamics is critical. It is imperative to work with expert moderators trained in qualitative research and implicit bias in order to create opportunities for marginalized communities to provide input in safe and effective ways. This is why it is important for industry to work with patient groups to create and facilitate their diversity and inclusion, patient council, and related initiatives, to co-create solutions across the clinical trial continuum.
Early, Often, and Throughout
Acknowledgements: The authors would like to thank the Savvy Cooperative participants in the workshop and panel for their courage and willingness to share their perspectives, and the BI planning committee, including Nicole Cohen, Nicole Forman and Lakisha Rodwell.

