Around the Globe
Amity Institute of Pharmacy, Amity University, India
International Pharmaceutical Quality, Sweden
Amity Institute of Pharmacy, Amity University, India
n the current innovation era and in the wake of COVID-19, two topics of rising importance for enabling patient access to newer treatment modalities are regulatory harmonization and drug-device combination products.
- Improved targeting of treatments,
- Enabling home treatment, and
- Realizing the potential for digital technologies to optimize and monitor clinical outcomes.
Innovative, integrated delivery devices are useful for vaccines as well as treatments required for cancer, heart disease, multiple sclerosis and many more serious and chronic diseases. Drug-device combination product types include the classic prefilled syringes and pens, auto-injectors, metered-dose inhalers, dry powder inhalers, and increasingly the inclusion of connected software. However, regulation of these products is complex.
Combination products (CP) such as these are governed by two or more different sets of regulations, based on their components and primary mode of action. This can lead to challenges for regulatory authorities to agree on product review jurisdiction and also how to streamline the drug and device requirements according to philosophy, documentation, and timelines.
Even within a single jurisdiction and regulatory agency things can get difficult. In the US, the Office of Combination Products (OCP) was set up in 2002 and has been active in producing regulations, guidance, training, and participating in external outreach activities. OCP team members participate in conferences to share their learning and their facilitation role for dealing with external queries and internal training, communication and coordination across divisions.
Moreover, around the world, convergence of regulations is handled in different ways and classifications are subject to different interpretations. Due to globalization, diversification of products, and the rising use of digital technologies in healthcare, challenges for both regulators and industry are set to increase.
While the International Council on Harmonization (ICH) has made great strides in the alignment of drug regulations over the past thirty years, and the International Medical Devices Regulators Forum (IMDRF) aspires to harmonize global medical device requirements, there is currently no international body to specifically address the additional complexities of trying to bring the two worlds of drug and device regulation together. The International Coalition of Medicines Regulatory Authorities (ICMRA) has recognized the challenges and opportunities at the interface of medicinal products and medical devices, but it may be the case that a completely new body, comprizing people with direct experience of combination products, could be the best solution for the future.
Harmonization Efforts in Asia-Pacific
In addition, across the region there are:
- Differences in clinical practice,
- No specific regulatory submission formats,
- Unclear submission processes, determined by the Primary Mode of Action (PMOA), and
- No lifecycle-management guidances.
Table 1 below illustrates this heterogeneous and challenging state of affairs for drug-device combination products.
#: Complex regulation to understand as all components applied
$: Applied as per PMOA
Table 1: Comparative regulations of combination products (CPs): Current status in selected countries in Asia-Pacific region.
| Singapore | Indonesia | Malaysia | Thailand | China | Australia | India | |
| Definition of CP |  |
 |
 |
 |
 * * |
 |
 |
| Legal status/PMOA |  |
 |
 |
 |
 * * |
 |
 # # |
| CP Committee for application review |  |
 |
 |
 |
 * * |
 |
 |
| Manufacturing /CMC regulations for CPs |  $ $ |
 $ $ |
 $ $ |
 # # |
 * * |
 $*# $*# |
 $ $ |
| Lifecycle management |  $# $# |
 $# $# |
 $# $# |
 $# $# |
 $# $# |
 *$# *$# |
 $# $# |
Asian Harmonization Working Party (AHWP)
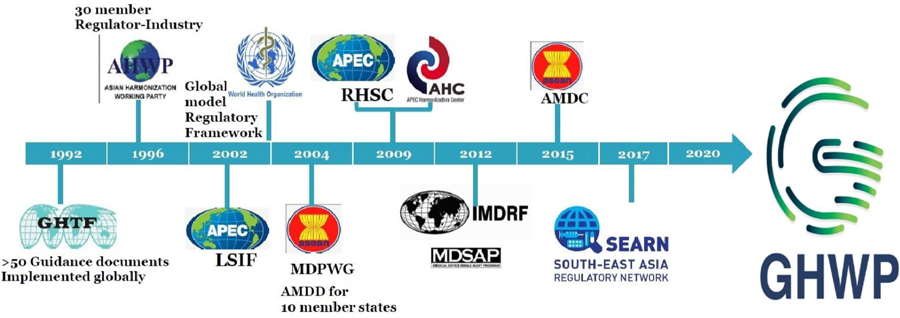
In 2015, the Asian Harmonization Working Party published a document outlining the development stages of several countries to demonstrate the different approaches to definitions, specific offices to deal with combination products, guidelines, etc. This useful and important document is referred to often in discussions addressing this topic. It highlighted several countries that are in the process of updating their regulations and guidances; the fact that much of the new guidance aligns with other more established regions, such as the US or Europe, is welcome.
Malaysia in particular has demonstrated a leading role for regulatory reliance by effectively working to collaborate across the medical device and medicinal products agencies (i.e., Medical Device Authority [MDA] and National Pharmaceutical Regulatory Agency [NPRA]) in Malaysia and communicated clearly to industry their plans to introduce new requirements and to help implement them.
A fundamental principle explained in the Malaysian 2019 guidance, aiming to make the process for evaluation of drug-device combination products more efficient, is recognition of previous product or component approvals in Australia, Canada, Europe, Japan, US, or the Medical Device Agency of Malaysia. This enables an abbreviated submission and review.
However, the overarching work on global harmonization for combination products initiated by AHWP seems to have stalled and, according to their website, in 2020 the group evolved into the GHWP (Global Harmonization Working Party). As yet, the website does not mention combination products, but this could potentially be a good opportunity for development of a specific group to work on this complex area.
Embracing the Future and Learning with Regulatory Evolution
Available expertise and resources in global regulatory agencies are also challenges in the alignment of regulation of drug-device combination products. It is not surprising that previous attempts to harmonize international requirements have not yet borne fruit, but the GHWP may hold some hope for future collaboration.
In addition to these complexities, the world has had to cope with the impact of COVID-19. However, arising from this global challenge has been acknowledgment that more regulatory collaboration, communication, and reliance across nations is not only vital but possible. For rare diseases, where the high cost of implementing a regulatory strategy for limited numbers of patients may prove prohibitive, alignment and improved efficiencies are essential.
Some small steps towards collaboration between medicines regulators, medical device regulators and industry experts are also being taken by international standards organizations and others, such as the ASTM standard on combination product terminology, being led by industry with regulator involvement–including the Gulf Healthcare Council–and the AAMI Risk management guidance for combination products, led by FDA’s Office of Combination Products with industry input.
With continued cooperation and collaboration from global regulators working with industry to address the rapidly developing challenges and opportunities, the future for smooth patient access to innovative drug-device combination products could be bright.

