The Promise and Problems of Real World Data and Evidence (RWD/RWE) for Patients and Companies
Patient Advocates In Research (PAIR)
Parexel Access
eal World Data (RWD) and Real World Evidence (RWE) are increasingly important to support product approvals and reimbursement for companies. They also have potential to help accelerate patient access to important new therapies. Patients, however, are more interested in Real World Answers (RWA) that must be rigorously and carefully studied in order to offer realistic value that translates into real improvements for patients, families, and healthcare systems.
RWD ≠ RWE ≠ RWA
The first step toward real value is to ensure that the terms are used in similar ways, so that proper expectations can be spread throughout the research, medical, and patient communities.
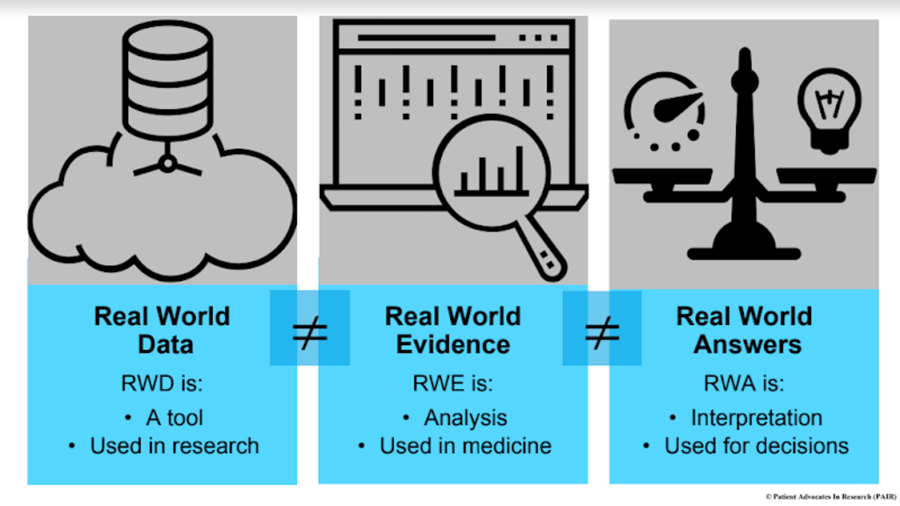
- RWD are raw data that come from routine healthcare settings, like electronic medical records (eMR), observational studies, patient registries, patient-reported data, mobile devices, and claims and billing data. The FDA further describes RWD as “data derived from sources other than traditional clinical trials.” RWD do not produce better patient results alone — they are a tool, similar to biospecimens.
- RWE, on the other hand, is the clinical evidence about the usage and potential benefits or risks of a medical product derived from analysis of RWD. RWE is generated when appropriate analytic techniques are applied to RWD to offer clues about what people and patients in healthcare settings will actually experience, during and from their care. In this way, RWE can provide meaningful insights to stakeholders if used well, informing clinical trials and reimbursement decisions, and supporting regulatory approval.
- RWAs, though, are the real goal: the insights and learnings that emerge when we interpret RWE in the context of patient care paths and experience, physician practice patterns, and even cultural and regional differences in healthcare settings. This transformation is the key, the opportunity to drive clinical practice changes and better patient results (known as outcomes to researchers), and to deliver what is most important to both patients and healthcare providers. To date, sponsors have not focused on these critical answers.
The Promise
Many sponsors are considering how RWE in clinical trials may lead to more adaptive trial designs, faster and less costly clinical trials, and faster approvals that may help reach broader populations. For example, synthetic control arms in phase 1, 2 and 3 clinical trials may offer accelerated trial timelines. By generating the control arm from existing data rather than by enrolling a second, comparator study arm, they may also eliminate the need to randomize trial participants to placebo treatment. This important change can make trials more appealing to patients, shorten timelines, and even allow for multiple options to be studied at the same time in platform trials.
RWE studies can help better capture information on patients’ true experience with their condition and their treatment, i.e., how treatment impacts their lives and well-being, beyond just “traditional” clinical measures. These studies can also help payers better understand the natural history and burden of an illness, particularly with rare diseases. This can provide a valuable foundation for securing patient reimbursement for important new therapies. Remember, patients and their families are payers too, giving sponsors another reason to collect this type of data.
The Problems to Solve
RWD, for instance, is only as good as the data source itself. A rigorous quality control process is needed to ensure that the data are accurate, timely, and useful. While offering an exciting alternative to collecting typical prospective data, Electronic Medical Records (eMRs), in particular, can pose major issues for researchers. Patients, for instance, often have multiple eMRs, depending on the cascade of physician offices they must work through on their way to a diagnosis. In addition, since the eMRs were not designed for research purposes (they stem from financial billing systems), errors and data gaps often exist. Patients often find these but cannot correct them, and even physicians in that system can be powerless to address the challenges. These errors can cause medical harm in some cases, but always create inaccuracies that researchers may not be aware of. It will take all of us to resolve this issue, and patient advocates are ready to work together with industry to do so.
The very way patients are approached to collect and share their data can be another complicating factor. Many patients feel they are treated as if they are their disease, and now feel that they are being viewed as simply a data “repository” rather than as a person. When the research mindset is “how much we can take from patients?”, this unintended message is received quite clearly by patients. If unfettered access to patient data is desired, it is incumbent upon researchers to focus on how patients can benefit from new technology or data-sharing practices before serving their own purposes.

