Around The Globe
African Medicines Agency Treaty Endorsed by African Union
What’s Next?
Senior Program Officer Regulatory Affairs, Africa Systems
Bill and Melinda Gates Foundation
he African Union (AU) approved the treaty for establishing the African Medicines Agency (AMA) on February 11 in Addis Ababa. This follows five years of consultations across the continent that were led by a task team established by the AU Commission and the World Health Organization (WHO).
Establishment of the AMA is based on AU Executive Council Decision EX.CL/Dec.857(XXVI) of January 2015 which:
- Recognized the need to strengthen the capacity for regulation of medical products in Africa, and the harmonization of medicines regulatory systems as a foundation for the establishment of a single regulatory Agency for Africa within the framework of the Pharmaceutical Manufacturing Plan for Africa.
- Endorsed the establishment of the African Medicines Agency (AMA).
- Requested the AUC, The New Partnership for Africa’s Development (NEPAD, the development agency of the African Union) and the WHO in collaboration with other stakeholders to define the scope of the medicines or medical products that would be covered by the work of the AMA, and to work out detailed modalities, institutional framework, legal and financial implications, of the establishment of the AMA.
Proposed Operating Model
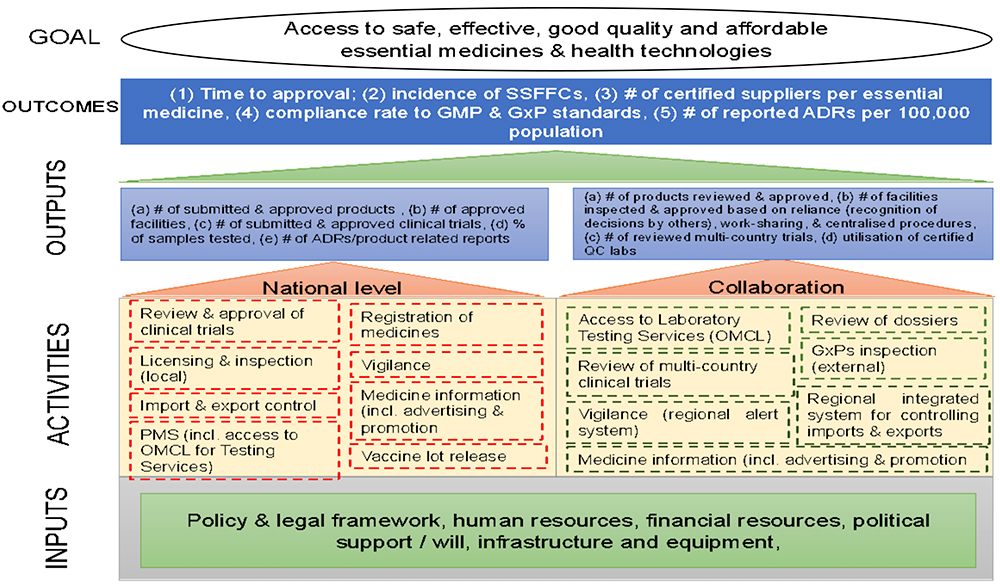
In the meantime, the AU Commission and NEPAD will develop the criteria for the basket of products and services that AMA will offer. It is expected that this will be a small group of products that merit assessment at a continental level due to their complexities, public health need, or other rationale (that will be defined). The bulk of medical products will continue to be assessed by national regulatory agencies. Other areas may include review of multi-country clinical trials, and inspections for AMA eligible products. Selection criteria for the Secretariat host country will also be developed.
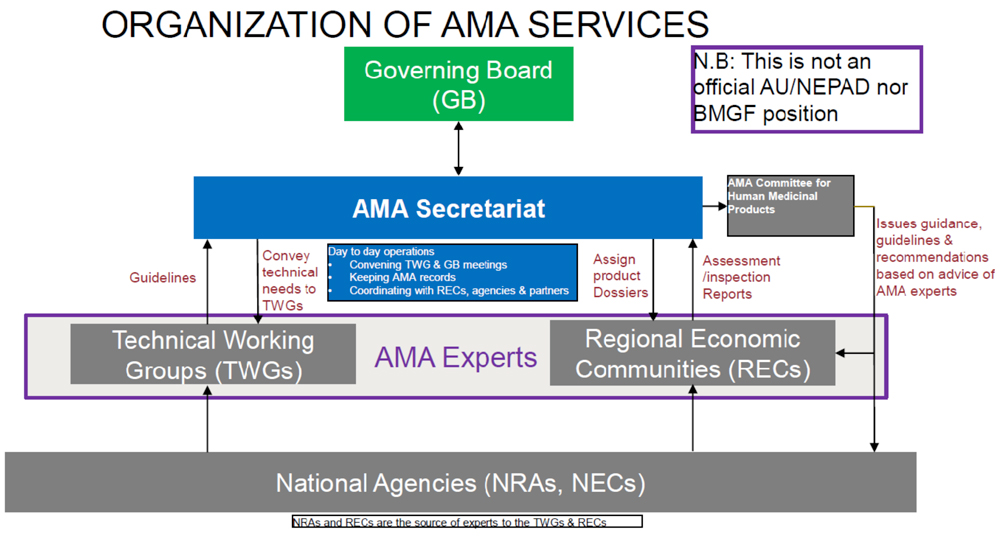
The expertise for AMA will be drawn from the member state regulatory authorities. AMA is expected to set standards, as well as provide regulatory recommendations that countries can rely on to make their decisions. Based on conversations we have had with NEPAD and other stakeholders, the organization of AMA services could look like this:
Other Africa Updates
In February 2019, the African Vaccine Regulatory Forum (AVAREF), a network of regulators and ethicists that seeks to optimize the clinical trial regulatory environment, met in Addis Ababa; at this meeting, CEPI and its Lassa fever vaccine development partners presented their development program, including plans to launch clinical studies in Africa.
The 4th Biennial Scientific Conference on Medicines Products Regulation in Africa (SCoMRA) will be held September 30 – October 1, 2019 in Victoria Falls, Zimbabwe. The theme of the conference is: “A Decade of regulatory harmonization in Africa: Where are we? Where do we go from here?” The call for abstracts will go out shortly. For more information about the conference, visit NEPAD online.

