How Important is FDA’s Payer Communication Guidance to Industry?
Sissi V. Pham
CEO
Aesara, Inc.
ne of the major windfalls for industry from the 21st Century Cures Act (section 3037 amendment) was the release of the US Food and Drug Administration (FDA) Payer Guidance document Drug and Device Manufacturer Communications With Payors, Formulary Committees and Similar Entities—Questions and Answers, which was finalized this year. The FDA Payer Guidance provided much needed clarity on the key requirements for payer communications for approved medical products and remarkably in the same document extended recommendations for pre-approval communications for population-based reimbursement and access decisions.
Key Takeaways
- For translational science (or the “bench-to-bedside” paradigm) to be realized fully in an environment where healthcare delivery is shifted from volume- to value-based, it is critical to demonstrate value for money by communicating the economic and health benefits of treatments.
- Proactive communication of relevant information for population-based reimbursement and access decisions to payers is low risk for industry.
- Industry needs to invest in health economics outcomes research (HEOR) and increase the flow of healthcare economic information (HCEI) for population-based healthcare access decisions by payers.
It has been twenty years since the first issuance of an amendment to the Food, Drug and Cosmetic Act (FD&C Act, section 502(a)), facilitating communication of HCEI to payers via the 1997 FDA Modernization Act (section 114; see Figure for timeline). Many of the questions industry and payers have had since the original amendment was released have been addressed in section 3037 of 21st Century Cures Act and the new FDA Payer Guidance. The FDA explicitly noted the important difference between information required for medical product approval and what is needed by payers to make decisions about population-based coverage and reimbursement. The FDA Payer Guidance is largely pragmatic regarding HCEI communications and advances industry’s proactive dialogue with payers.
Communication with Payers: Legislation and Guidance Activities
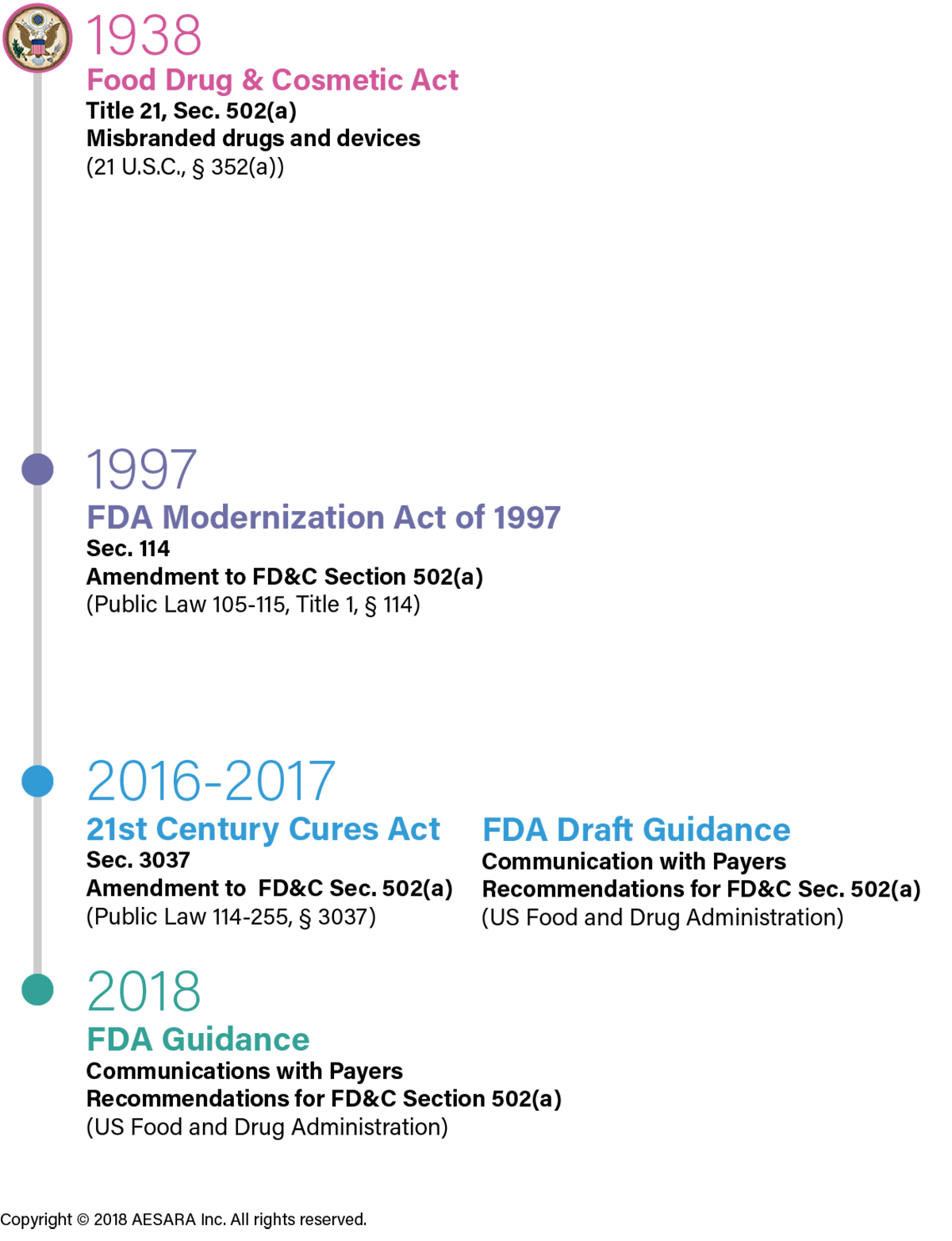
Chronology of federal statutes leading up to the new FDA Payer Guidance.
Opportunities
The FDA Payer Guidance has generally been well received by both industry and payers, and it offers great opportunity to bring advances in medical products to the patient. The impact and value of new medical products, however, should be collaboratively researched at the healthcare system level to answer critical questions, such as: What are the appropriate quality outcomes for payers and employers? When is access cost-effective or affordable for different stakeholders? What is the cost impact of implementing certain practice guidelines for policymakers? Communicating this evidence transparently to payers via the HCEI 502(a) (section 502(a) of FD&C Act) avenue will foster use of products with proven health benefits and facilitate access to such products for healthcare systems and patients.
Interestingly, the FDA expounded on ways that HCEI 502(a) materials could be presented and specified an “evidence dossier” in addition to other expected means of communication. Until now, evidence dossiers have been issued by industry only in response to unsolicited requests from payers, often in the form of the Academy of Managed Care Pharmacy (AMCP) Dossier. It appears that dossiers containing HCEI evidence related to the indication of the medical product that is based on competent and reliable scientific standards can now be used proactively with payers.
Although section 502(a) of the FD&C Act focused on the misbranding of approved drugs and devices, the new FDA Payer Guidance unexpectedly provides guidance for communicating information about unapproved medical products and their uses to payers as a means of facilitating “future coverage and/or reimbursement decisions prior to FDA approval, clearance, or licensure” – as long as there is no characterization of safety or effectiveness of the specific product. The FDA takes it a step further in stating that, if industry follows the recommendations to provide factual, pre-approval medical product information, the “FDA believes that the risk that payers will be misled is relatively low.”
With the recommendations in the FDA Payer Guidance, coupled with the fact that no known notice of violations or warning letters has been issued related to health economic communications to payers, it is reasonable to surmise that communicating information relevant for population-based reimbursement and access decisions to payers is low risk for industry and will not be of significant concern for the FDA. In addition, the simultaneous release of the guidance on Medical Product Communications That Are Consistent With FDA-Required Labeling alongside the FDA Payer Guidance provides further insight into how to effectively and compliantly communicate externally. It’s noteworthy that payers already scrutinize medical products and essentially regulate their usage through robust processes to approve reimbursement and access. As such, both payers and the FDA serve as gatekeepers and share the same mission to advance the health of patients.
Challenges
With the FDA Payer Guidance now available and much of the uncertainty about communication to payers clarified, one might expect an upsurge in proactive communications with payers about unapproved medical products. However, this may not necessarily occur. For many drug and device companies, product registration remains paramount, and research related to market access is a secondary concern when it should in fact be parallel with the goal of achieving regulatory approval. As a result, research to generate HCEI is not routinely conducted during drug development or post-approval, nor prioritized for external communication. A related challenge is the general lack of understanding of the relatively new discipline of HEOR (health economics outcomes research) that generates HCEI. Experienced HEOR researchers should be involved early and be integrated into medical product development to identify the most appropriate endpoints for clinical trials, conduct real-world observational studies, and develop economic models to project financial impact. Without these goals incorporated into product development, compelling HCEI is not assembled and opportunity for payer reimbursement and market access can be substantially reduced or lost. In addition, to ease the internal approval challenges of such materials the review committees within drug and device companies will need to have a better grasp of the fundamental HEOR methods behind HCEI and to have more insight into payer decision processes to understand the significance of HCEI 502(a) materials for value-based reimbursement decisions and access negotiations.
Conclusion
Economic stability for the healthcare system is critical to ensure patients have access to beneficial medical products. Given the growing constraints in healthcare funding, it is essential for industry to come well prepared to payers with sound healthcare economic evidence and access solutions for patients. To achieve this goal, industry will need to invest in HEOR and increase the flow of HCEI for population-based, healthcare access decisions. The FDA has issued sensible guidances for communication, creating a clearer and safe pathway for such payer interactions, and now industry needs to leverage the opportunity fully.
References available upon request.

