Chair, DIA Board of Directors

DIA Global Chief Executive
marked a year of tremendous advancements for our association. Thank YOU. You showed unwavering commitment to our mission and lent your voice to our shared quest to drive progress against the challenges in healthcare product development.
Every day, somewhere around the world, members of the DIA community are taking the next step, passing the baton, and creating new knowledge as we collectively move forward in our mission to advance the health and well-being of the patients who inspire us. You make extraordinary progress possible.




are a global association that mobilizes life science professionals from around the globe and across all areas of expertise to engage with patients, peers, and other experts in a neutral environment on the issues of today and the possibilities of tomorrow.

Engaging Patients
DIA has been a pioneer in advocating for the patient voice for more than a decade. Since the launch of its first Patient Fellowship in 2006, DIA has brought patients to the table as equal stakeholders in numerous global patient engagement initiatives, providing fertile ground for fruitful collaborations among patients, researchers, industry, payers, and regulators.
s the call for meaning- and impactful patient engagement grows stronger, so does the push for an increasingly empirical approach to address the topic. In 2017, we continued to drive the movement towards evidence-based patient centricity and engagement across the entire healthcare ecosystem.
- We released the first findings of the 2016 multi-phase research study on patient-centric initiatives in drug development conducted in partnership with Tufts University.
- A Patient Group Advisory Council (PGAC) was established to provide a patient perspective on DIA’s patient engagement strategies
- Patients, and other stakeholders, shared fresh, multi-faceted insights on meaningful patient engagement at three key DIA events.
- Most recently, DIA entered into a collaboration with more than 30 patient-focused organizations via the International Leadership Group (PILG) of the new Innovative Medicine Initiative (IMI) PARADIGM (Patients Active in Research and Dialogues for an Improved Generation of Medicines).
Why we care: Patients have an irrefutable role in shaping the care they need; involving patients throughout the healthcare product development lifecycle helps drive better design and development, and faster approval, of therapies that deliver meaningful health improvements.
Advancing Therapies from Bench to Bedside
ast year, DIA continued to provide a unique forum for advancing the field of translational science by collaborating and exchanging fresh insights with health authorities, industry, regulators, patients, and academics.
- Together with the National Center for Advancing Translational Sciences (NIH-NCATS) we convened a group of industry clinical development experts to provide input into NCATS’ Trial Innovation Network.
- Together with the National Center for Advancing Translational Sciences (NIH-NCATS) we convened a group of industry clinical development experts to provide input into NCATS’ Trial Innovation Network.
- Our active DIA Clinical Trial Disclosure Community addressed questions related to the implementation of recent Clinical Trial Disclosure guidelines, including the EMA Policy 0070 and the US Final Rule.
- We convened a Rare Diseases Town Hall at our 2017 Global Annual Meeting to address challenges specific to orphan drug development, and published a White Paper in the American Heart Journal that examines methods to collect social listening data for use in cardiac safety surveillance.
- We explored ways to accelerate the translation of basic research to clinical application in China at the 3rd DIA China Drug Discovery Innovation Conference.
- The 14th DIA Japan Annual Meeting focused on advancing the field of genomic medicine and the development of next-generation medicines through big data and artificial intelligence.
- Our active DIA Clinical Trial Disclosure Community addressed questions related to the implementation of recent Clinical Trial Disclosure guidelines, including the EMA Policy 0070 and the US Final Rule.
- We convened a Rare Diseases Town Hall at our 2017 Global Annual Meeting to address challenges specific to orphan drug development, and published a White Paper in the American Heart Journal that examines methods to collect social listening data for use in cardiac safety surveillance.
- We explored ways to accelerate the translation of basic research to clinical application in China at the 3rd DIA China Drug Discovery Innovation Conference.
- The 14th DIA Japan Annual Meeting focused on advancing the field of genomic medicine and the development of next-generation medicines through big data and artificial intelligence.

Why we care: Translational science turns laboratory, clinical, and community findings into interventions that improve the health of individuals and the public. DIA works closely with researchers at the forefront of this rapidly advancing field to break through roadblocks and accelerate therapies from bench to bedside.
Advancing Regulatory Science
his past year, DIA continued to support the global evolution of regulatory science to increase efficiency through regulatory convergence, and the development of data and submission standards:
- DIA was named Authorized Training Partner of the ICH (International Council for Harmonization), offering ICH-approved courses in alignment with the latest ICH guidelines.
- DIA was named Authorized Training Partner of the ICH (International Council for Harmonization), offering ICH-approved courses in alignment with the latest ICH guidelines.
- Following recommendations from the DIA Council of Regulators (COR), we addressed current regulatory challenges, including ICH training needs and the use of Artificial Intelligence (AI) and Real World Data (RWD) in regulatory decision making.
- As a prelude to China’s official ICH membership announced in June 2017, we successfully collaborated with the China Food and Drug Administration (CFDA) to arrange a joint ICH workshop in conjunction with the 9th DIA China Annual Meeting.
- Several DIA meetings, including the 2017 Joint International Conference on Clinical Trials in Korea, the 14th DIA Japan Annual Meeting, and the DIA 2017 Global Annual Meeting, highlighted the growing role of AI in healthcare and the regulatory approval process.
- We successfully collaborated with India’s Central Drugs Standard Control Organization (CDSCO), FDA, and EMA to host a 2-day USFDA-EMA-CDSCO-DIA Multicenter GCP Workshop in India.
- In Japan, we co-hosted for the first time the 12th Summit of Heads of Medicines Regulatory Agencies and the International Coalition of Medicines Regulatory Authorities (ICMRA).
- Following recommendations from the DIA Council of Regulators (COR), we addressed current regulatory challenges, including ICH training needs and the use of Artificial Intelligence (AI) and Real World Data (RWD) in regulatory decision making.
- As a prelude to China’s official ICH membership announced in June 2017, we successfully collaborated with the China Food and Drug Administration (CFDA) to arrange a joint ICH workshop in conjunction with the 9th DIA China Annual Meeting.
- Several DIA meetings, including the 2017 Joint International Conference on Clinical Trials in Korea, the 14th DIA Japan Annual Meeting, and the DIA 2017 Global Annual Meeting, highlighted the growing role of AI in healthcare and the regulatory approval process.
- We successfully collaborated with India’s Central Drugs Standard Control Organization (CDSCO), FDA, and EMA to host a 2-day USFDA-EMA-CDSCO-DIA Multicenter GCP Workshop in India.
- In Japan, we co-hosted for the first time the 12th Summit of Heads of Medicines Regulatory Agencies and the International Coalition of Medicines Regulatory Authorities (ICMRA).

Opening ceremony, 9th DIA China Annual Meeting 2017

Panel discussion, DIA 2017 Global Annual Meeting
Why we care: To accelerate the delivery of safe and more efficient therapies to patients around the world, it is essential to advance the tools, standards, and approaches involved in the development, manufacturing, evaluation, and regulation of these therapies. We aims to advance the evolution of regulatory decision-making by helping identify key questions and priorities that exist in today’s global regulatory system.
Better Value, Quicker Access
n 2017, we continued to bring global payers, industry leaders, clinicians, regulators, patients, HTAs, and other stakeholders together to work towards our overarching goal of bringing high-quality medicines to patients faster:
- Together with the Boston Consulting Group (BCG) we held two focus groups and one Market Access Roundtable about the sustainability of healthcare funding to develop a common set of standards for future market access competencies.
- Together with the Boston Consulting Group (BCG) we held two focus groups and one Market Access Roundtable about the sustainability of healthcare funding to develop a common set of standards for future market access competencies.
- Our Value, Access, and Regulatory Strategy Workshop in Basel addressed the fragmented healthcare landscape in Europe by focusing on approaches that could more effectively align regulatory and reimbursement strategies.
- We collaborated with Pharmaphorum and Vital Transformation on two webinars exploring access challenges and solutions in the changing European healthcare market: Approval and Access: Overcoming the Final Hurdle of Drug Development and Can Europe Bring the Next Generation of Biotech Therapies to Market?
Why we care: As medicines become increasingly innovative and sophisticated, rising manufacturing costs often render life-saving treatments out of reach for patients. The question of how to expedite patient access to affordable, high-quality healthcare is particularly pressing in an ecosystem where stakeholder goals such as cost containment, profitability, high quality, timely access, and affordability are frequently at odds with each other.
- Our Value, Access, and Regulatory Strategy Workshop in Basel addressed the fragmented healthcare landscape in Europe by focusing on approaches that could more effectively align regulatory and reimbursement strategies.
- We collaborated with Pharmaphorum and Vital Transformation on two webinars exploring access challenges and solutions in the changing European healthcare market: Approval and Access: Overcoming the Final Hurdle of Drug Development and Can Europe Bring the Next Generation of Biotech Therapies to Market?
Why we care: As medicines become increasingly innovative and sophisticated, rising manufacturing costs often render life-saving treatments out of reach for patients. The question of how to expedite patient access to affordable, high-quality healthcare is particularly pressing in an ecosystem where stakeholder goals such as cost containment, profitability, high quality, timely access, and affordability are frequently at odds with each other.
Learning with DIA
op companies around the world entrust us with their staff to receive professional training that is current, effective, and relevant. Among the most highly valued DIA events are individually tailored, in-house courses designed to meet the unique needs of each company and its staff.
In 2017, we continued to offer both face-to-face, online, and blended learning solutions to thousands of professionals worldwide. In addition, we launched three new, self-paced, online eLearning programs and one blended course that combines online digital media with traditional classroom time and other activities:
- The online, self-paced Medical Affairs eLearning Program consists of 11 modules that cover key elements for success in Medical Affairs, including the role of the Medical Science Liaison, safety reporting, stakeholder engagement, development of publications, compliance, and actions for medical information professionals.
- The Good Clinical Practice eLearning Module uses an interactive case study to teach participants how to conduct clinical research studies that comply with regulations, including recent ICH guidelines.
- The newly redesigned Drug Safety eLearning Program covers a comprehensive list of topics that range from regulations and requirements to pre-market review and post-market monitoring.
- In the Drug Safety and Pharmacovigilance across the Product Lifecycle course, participants learn about current principles, regulatory expectations, and practical approaches to drug safety and pharmacovigilance in key regulatory jurisdictions. This is a comprehensive blended course that combines online learning, instructional sessions, and real-world case studies with hands-on interactive exercises.
Why we care: At every stage of their career, healthcare professionals have to stay abreast of critical issues spanning the healthcare product life cycle. Now more than ever, it is critical to offer professional training that is current, effective, relevant, and trusted.

Members learn about FDA regulations and expectations in a mock FDA meeting
For more than half a century, we have provided learning solutions to healthcare professionals worldwide. We offer highly effective, customized training programs ranging from live and online instructor-led courses to self-paced eLearning formats, including officially accredited continuing education programs.
Making Connections
cross corporate, regulatory, and international boundaries, DIA’s Communities and Working Groups serve as easily accessible hubs for stimulating, peer-to-peer discussions, professional networking opportunities, lively idea exchanges, and high-quality content created by our members.
- In 2017, we added seven new working groups across the currently more than 20 DIA Communities.
- On July 4, 2017, DIA Japan held its 1st DIA Japan Community Day, subtitled “I have learned everything necessary for life in the DIA Community.”
- Re-energized in 2017, the Devices and Diagnostics Community put forward an outstanding activity schedule for 2018 that includes webinars, presentations, and thought-provoking discussions on cutting-edge topics affecting the field.
- The recently launched DIA Grand Challenges Program allows DIA Community members to identify the most pressing problems in their respective fields and work together to find solutions that move both the field and the global healthcare industry forward. Results will be announced mid-2018.
- The Leader of Tomorrow Program and its accompanying App were piloted in Europe in 2017 to engage students and emerging professionals in networking and career development. The program will expand globally in 2018.

Why this matters: DIA Communities offer our members exclusive access to global conversations around healthcare. DIA members work together to speed innovation in healthcare product development, producing resources such as tools, case studies, and best practices that benefit the entire healthcare ecosystem.
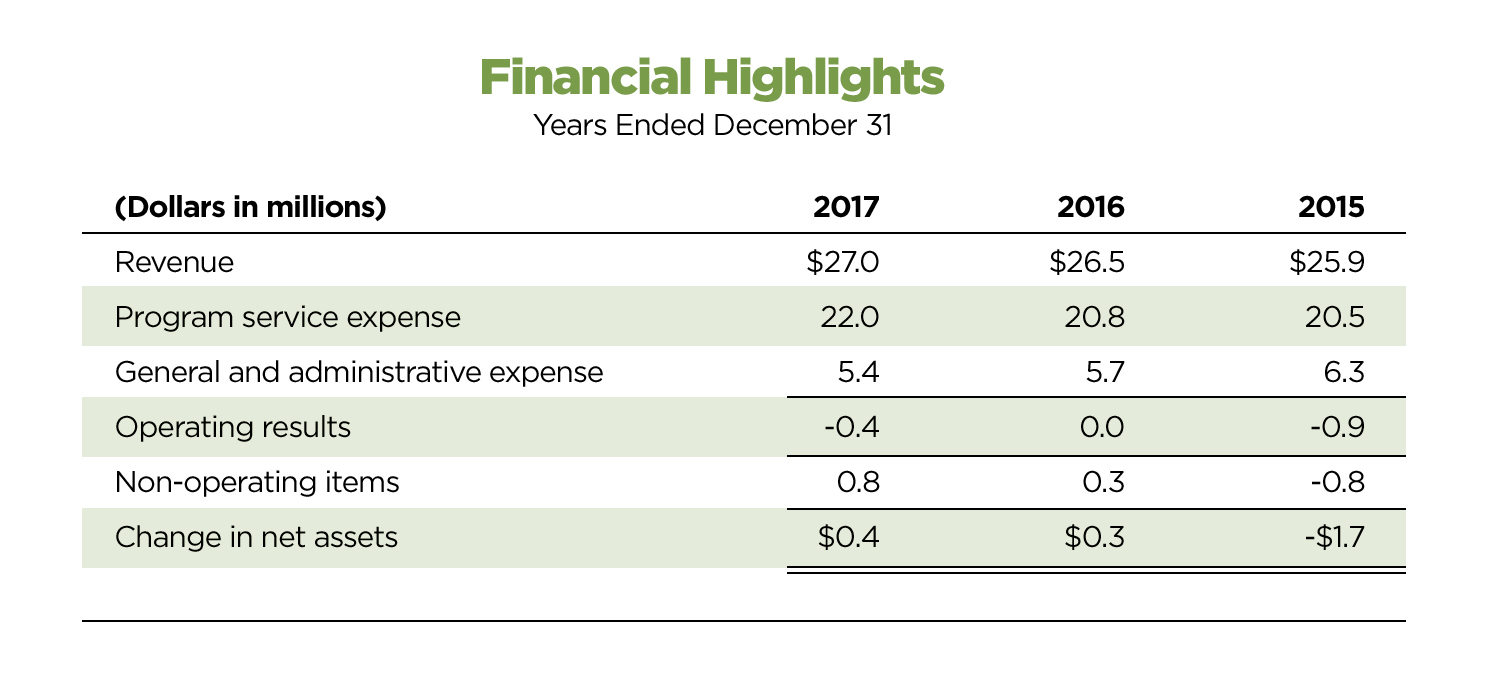
DIA Financials
or the year ending December 2017, DIA delivered a contribution to operating financial results, as well as a positive change in net assets.