Kanagawa Dental University, Japan
National University of Pharmacy, Ukraine
Good Clinical Practice Alliance – Europe (GCPA)
Strategic Initiative for Developing Capacity in Ethical Review (SIDCER)
@SIDCER_EU
ivilized society has constructed rules and protective measures to prevent repeating grievous human mistakes and to protect human health, safety, and rights. These include the CIOMS (Council for International Organizations of Medical Sciences) health research guidance on ethics, medical product development, and safety, the Declaration of Helsinki, and other guidelines which declare protection of life, human rights, and diversity of cultures.
Many current guiding codes of ethics were established after detailed analysis and wide discussion of research that was clearly unethical and unacceptable. However, medical research in countries where military conflicts are taking place requires additional ethical attention and careful consideration. Previous human experience has shown that there is a high probability of developing negative tendencies and even unethical abuses in such situations. However, the research situation in Ukraine during this war is unlike previous ones. No one would say that the research itself is unethical. Nonetheless, these research studies are now taking place in a new challenging situation. We must reevaluate these projects taking into consideration the ongoing war.
General Ethical Considerations in Clinical Research
Informed consent is the primary building block of ethical clinical trial conduct. It codifies the concept that it is acceptable to experiment on someone if that person agrees to the experimentation. This practice was later refined: Informed consent in and of itself is not sufficient; there must also be an independent review to confirm that the research and the participation of those patients, even with their consent, would be ethical. This introduced the concept and process of ethics review by institutional review boards or research ethics committees.
The international medical and ethics communities came together as the World Medical Association to refine this concept. The WMA Declaration of Helsinki clearly states that the reason we do research is to continually improve our knowledge and tools for responding to disease, suffering, and the healthcare needs of individuals and populations (Article 6). This is one of the justifications, if not the most important one: that the results of the research will be used to improve the medical community’s response to disease, suffering, and healthcare. Medical research on other people cannot be carried out simply for scientific curiosity or because there’s something we want to know. The justification for experimenting on another human being is that it will help future patients in that similar situation.
The Ukraine Clinical Research Support Initiative (UCRSI)

Research Ethics Committees (RECs) in Ukraine
The war in Ukraine brings unprecedented operational challenges to clinical research ethics. Electronic communications tools have not been completely implemented, so there is no strong interaction between RECs. RECs in Ukraine must conduct ethical monitoring applying mostly remote methods. Organizing REC meetings and communications is difficult. Procedures must not only ensure the safety of committee members (who are all unpaid volunteers). They must also consider the need to ensure clinical trial data integrity and confidentiality as well as that review deadlines are met, in conditions lacking electronic data flow.
Other challenges for local RECs in ethics review and monitoring in Ukraine are related to the committee members’ lack of experience in working under bomb and missile attacks and energy lockdowns. Regardless, RECs have to constantly evaluate risks to their committee members, study participants, site personnel, clinical data quality, and confidentiality, and generally ensure proper human subject protection remotely in difficult circumstances.
Ethical Uncertainty Presents Complex Dilemmas
The most acute issues for RECs: What is the best way to monitor and protect research subject rights in trial sites in Ukraine that are particularly challenged, inaccessible, or impaired? How should interactions between RECs and clinical sites hosting patients from trials in other locations in Ukraine or even abroad be managed? These hosting sites give these subjects help. But should Ukrainian RECs continue to manage and protect their rights?
Rules of Research, Rules of War
We must establish a relationship between the rules of war and the rules of research. Informed consent is really about the relationship between the research participant, the researcher, and the sponsor. We must figure out how to care for that relationship and bring it forward through the disruptive, destructive environment of war.
It’s important to have research for health in a peaceful situation. But it’s also important to have research for health in a conflict situation.
Mapping the Ethical Terrain of Research during Armed Conflict
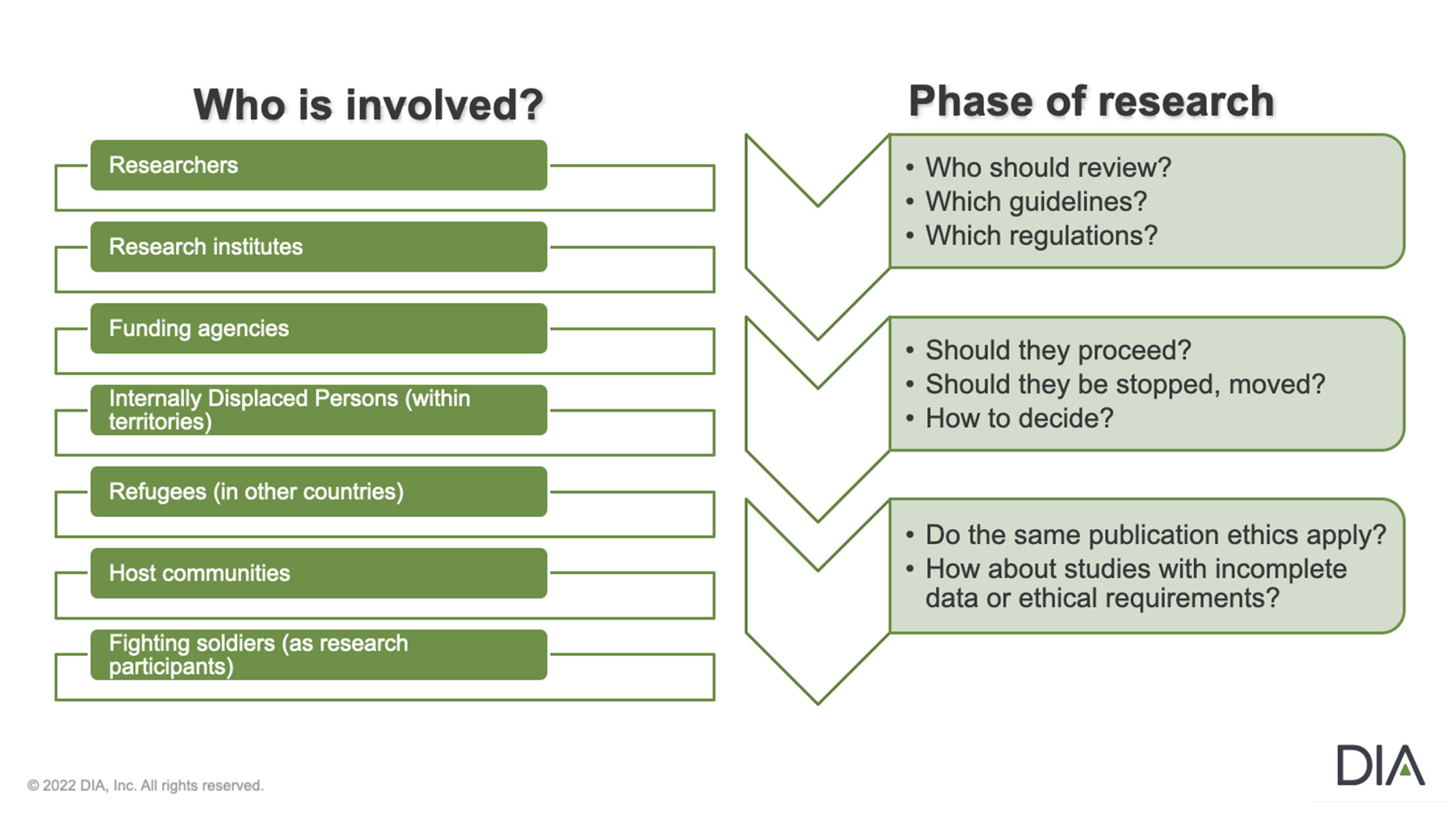
What Happens After the War?
The current conflict demonstrates an ethical responsibility for our international community to support this research enterprise during and after the war. How do we respond to this responsibility to research patients and to this enterprise? Can we create national and regional REC and IRB networks in times of peace so these networks can buttress and support RECs and IRBs conducting research during a conflict? Can we refine the informed consent framework that protects research participants in peacetime to provide similar information and protection during war?
To ensure ethical research continuity during times of disruption, all stakeholders in the global clinical trials and research enterprise must lead the way in revising guidance and other documents and providing direction to clearly allow and encourage continuity, which will preserve progress for future generations.

