Meeting Highlights: DIA China 2021
National Cancer Center / Cancer Hospital, Chinese Academy of Medical Sciences
ince China implemented the filing system of drug clinical trial institutions in December 2019, the capacity of clinical trials in medical institutions has been steadily upgraded and a large number of new sites have emerged. According to the National Medical Products Administration (NMPA) database, the total number of drug clinical trial sites registered nationwide exceeded 1,000 by the end of 2020. Improving the quality and efficiency of their own trials by improving the knowledge and training of their study staff, and integrating into the global drug development process, have become key issues for Chinese clinical trial sites.
In addition to tips for developing clinical trial sites for drug studies, the Patient-Centered Clinical Study Needs and Practice session at DIA China 2021 focused on numerous hot topics facing clinical sites and research professionals in China.
Clinical Cancer Needs
Ethics Reviews
Sponsor-Site Communication
Managing Investigator-Initiated Trials (IITs)
Patient-Centric Clinical Trials
Future Orientation of Research and Research Professionals
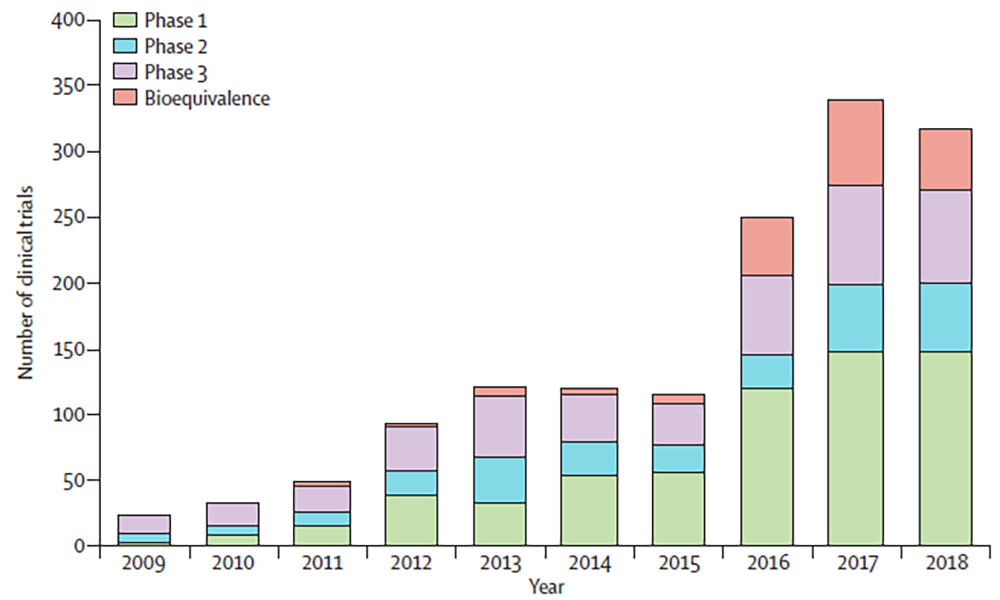
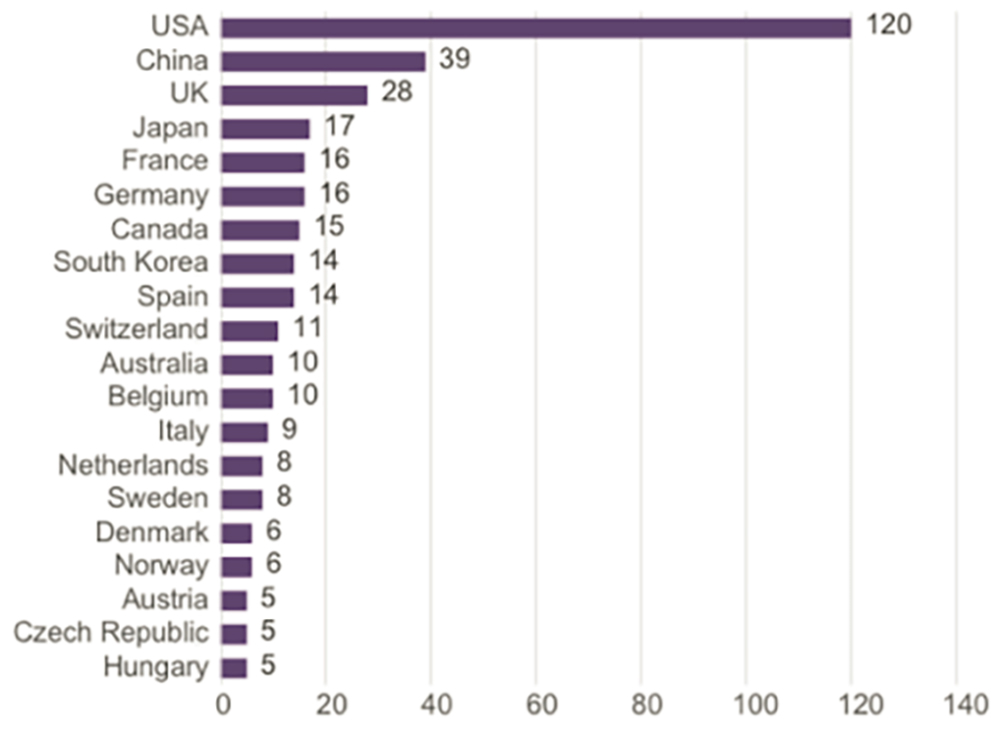
NMPA recently approved China’s first cellular product (CD19 CAR-T), and the total number of clinical trials in China for solid tumor cell or gene therapies ranked second in the world from 2010 to 2019 (Figure 2). The focus of research into cellular and gene therapies has been shifting from hematologic malignancies to solid tumors, and clinical development in China in the field of solid tumors is expected to keep pace with that of the US.
Well-designed drug development pathways result from the collaboration of sponsors, investigators, ethics committees, and patients, a network in which the study site and attending research professionals play a key connecting role. Many clinical sites are also recognizing that clinical research is both a special science and a professional discipline and are pursuing the benefits of professional development pathways. Full-time clinical trial physicians, pharmacists, nurses, and managers are emerging in Chinese clinical sites. Comprehensive trainings covering trial execution, management, design, statistical analysis, and clinical pharmacology are provided by clinical sites for investigators, and by medical schools for students.
The growing importance of medical and academic institutions in the future of clinical research in China was illustrated in various ways at DIA China 2021:
- Estimated 10% of meeting participants came from academic institutions
- 60 of these academic institution participants served as speakers
- 27 medical institutions participated in the meeting exhibition
- The Patient-Centered Clinical Study Needs and Practice session attracted more than 1,000 attendees and became a “meeting hotspot.”